| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:27:27 UTC |
|---|
| Update Date | 2020-06-04 20:40:35 UTC |
|---|
| BMDB ID | BMDB0000253 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
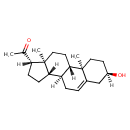
| Common Name | Pregnenolone |
|---|
| Description | Pregnenolone belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. Pregnenolone is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C21H32O2 |
|---|
| Average Molecular Weight | 316.4776 |
|---|
| Monoisotopic Molecular Weight | 316.240230268 |
|---|
| IUPAC Name | 1-[(1S,2R,5R,10S,11S,14S,15S)-5-hydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-14-yl]ethan-1-one |
|---|
| Traditional Name | pregnenolone |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@]([H])(O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)C(C)=O |
|---|
| InChI Identifier | InChI=1S/C21H32O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4,15-19,23H,5-12H2,1-3H3/t15-,16+,17-,18+,19+,20+,21-/m1/s1 |
|---|
| InChI Key | ORNBQBCIOKFOEO-QYYVTAPASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- 3-alpha-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Alcohol
- Organooxygen compound
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Endoplasmic reticulum
- Membrane
- Mitochondria
- Myelin sheath
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00kb-0292000000-b3b4cfe688e02f59e5d3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0a59-3910000000-99565a6755a481589ecb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0536-9800000000-92b51da359905f0f85c5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0095000000-95cc4f9c6ec45bf313eb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kb-0391000000-02f4b7354dc726dcdf6c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kbu-2490000000-0c314b18c977fbb00283 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0029000000-0f4f3e0717e63843600b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0079000000-6230ba3db61311fd6b87 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0592-1090000000-ef932dad775d120089d6 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, CDCl3, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CD3OD, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Shingate, Bapurao B.; Hazra, Braja G.; Pore, Vandana S.; Gonnade, Rajesh G.; Bhadbhade, Mohan M. Stereoselective syntheses of 20-epi cholanic acid derivatives from 16-dehydropregnenolone acetate. Tetrahedron (2007), 63(25), 5622-5635. |
|---|