| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:28:55 UTC |
|---|
| Update Date | 2020-05-11 20:08:17 UTC |
|---|
| BMDB ID | BMDB0000340 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3a,7a,12a,19-Tetrahydroxy-5b-cholanoic acid |
|---|
| Description | 3a,7a,12a,19-Tetrahydroxy-5b-cholanoic acid, also known as 3,7,12,19-thca, belongs to the class of organic compounds known as tetrahydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears four hydroxyl groups. Based on a literature review a significant number of articles have been published on 3a,7a,12a,19-Tetrahydroxy-5b-cholanoic acid. |
|---|
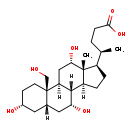
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3a,7a,12a,19-Tetrahydroxy-5b-cholanoate | Generator | | 3,7,12,19-THCA | MeSH | | 3,7,12,19-Tetrahydroxycholanoic acid | MeSH | | 3a,7a,12a,19-Tetrahydroxy-5b-cholan-24-Oate | HMDB | | 3a,7a,12a,19-Tetrahydroxy-5b-cholan-24-Oic acid | HMDB | | (4R)-4-[(1S,2R,5R,7S,9R,10R,11S,14R,15R,16S)-5,9,16-Trihydroxy-2-(hydroxymethyl)-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoate | Generator, HMDB | | 3alpha,7alpha,12alpha,19-Tetrahydroxy-5beta-cholan-24-Oic acid | MeSH, HMDB |
|
|---|
| Chemical Formula | C24H40O6 |
|---|
| Average Molecular Weight | 424.5708 |
|---|
| Monoisotopic Molecular Weight | 424.282489012 |
|---|
| IUPAC Name | (4R)-4-[(1S,2R,5R,7S,9R,10R,11S,14R,15R,16S)-5,9,16-trihydroxy-2-(hydroxymethyl)-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoic acid |
|---|
| Traditional Name | (4R)-4-[(1S,2R,5R,7S,9R,10R,11S,14R,15R,16S)-5,9,16-trihydroxy-2-(hydroxymethyl)-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoic acid |
|---|
| CAS Registry Number | 171524-64-4 |
|---|
| SMILES | [H][C@@]12CC[C@H]([C@H](C)CCC(O)=O)[C@@]1(C)[C@@H](O)C[C@@]1([H])[C@@]2([H])[C@H](O)C[C@]2([H])C[C@H](O)CC[C@]12CO |
|---|
| InChI Identifier | InChI=1S/C24H40O6/c1-13(3-6-21(29)30)16-4-5-17-22-18(11-20(28)23(16,17)2)24(12-25)8-7-15(26)9-14(24)10-19(22)27/h13-20,22,25-28H,3-12H2,1-2H3,(H,29,30)/t13-,14+,15-,16-,17+,18+,19-,20+,22+,23-,24-/m1/s1 |
|---|
| InChI Key | RFSKRKUKTCLIAV-OWXKUJNZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears four hydroxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Tetrahydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydroxy bile acid, alcohol, or derivatives
- 19-hydroxysteroid
- 3-hydroxysteroid
- 12-hydroxysteroid
- 7-hydroxysteroid
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Polyol
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Organic oxygen compound
- Primary alcohol
- Carbonyl group
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0bu3-0569200000-c724deddea0794c171b6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-004i-2100159000-6e1fcf87c606060d30cc | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0009600000-70600db74b6acb61211c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0009100000-49c2cffe03e2e60f22fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002k-1109000000-ccd4aca8f0bff807a8e6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-05fr-0003900000-8adfc90518b7a32e1a65 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-1009700000-30bd509011b013a0bb85 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-7009000000-3b2d081017bbf7ce399a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0570-0008900000-1257dece5f78104794f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0550-1009100000-69f08f15b2ecae209b5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-9770000000-c6a1204ae4e44ba4c905 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-fd0b098a165473974103 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0002900000-522cde860acb86cd80f5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004m-0019100000-49bd77c03794201e6eaa | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Kurosawa, Takao; Nomura, Yukihiro; Mahara, Reijiro; Yoshimura, Teruki; Kimura, Akihiko; Ikegawa, Shigeo; Tohma, Masahiko. Synthesis of 19-hydroxylated bile acids and identification of 3a,7a,12a,19-tetrahydroxy-5b-cholan-24-oic acid in human neonatal urine. Chem Pharm Bull (Tokyo). 1995 Sep;43(9):1551-7. |
|---|