| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:31:17 UTC |
|---|
| Update Date | 2020-05-11 20:20:58 UTC |
|---|
| BMDB ID | BMDB0000476 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
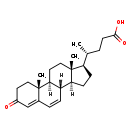
| Common Name | 3-Oxo-4,6-choladienoic acid |
|---|
| Description | 3-Oxo-4,6-choladienoic acid, also known as 3-oxo-4,6-choladien-24-Oate, belongs to the class of organic compounds known as bile acids, alcohols and derivatives. These are organic compounds containing an alcohol or acid derivative of cholic acid. 3-Oxo-4,6-choladienoic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-oxo-4,6-Choladienoate | Generator | | 3-oxo-4,6-Choladien-24-Oate | HMDB | | 3-oxo-4,6-Choladien-24-Oic acid | HMDB | | 3-Oxochola-4,6-dien-24-Oate | HMDB | | (4R)-4-[(1S,2R,10S,11S,14R,15R)-2,15-Dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-6,8-dien-14-yl]pentanoate | HMDB | | 3-oxo-4,6-Choladienoic acid | ChEBI |
|
|---|
| Chemical Formula | C24H34O3 |
|---|
| Average Molecular Weight | 370.525 |
|---|
| Monoisotopic Molecular Weight | 370.250794954 |
|---|
| IUPAC Name | (4R)-4-[(1S,2R,10S,11S,14R,15R)-2,15-dimethyl-5-oxotetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-6,8-dien-14-yl]pentanoic acid |
|---|
| Traditional Name | 3-oxo-4,6-choladienoic acid |
|---|
| CAS Registry Number | 88179-71-9 |
|---|
| SMILES | [H][C@@]12CC[C@H]([C@H](C)CCC(O)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])C=CC2=CC(=O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C24H34O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h5-6,14-15,18-21H,4,7-13H2,1-3H3,(H,26,27)/t15-,18+,19-,20+,21+,23+,24-/m1/s1 |
|---|
| InChI Key | CREVIXFSUWYGRJ-IHMUCKAYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bile acids, alcohols and derivatives. These are organic compounds containing an alcohol or acid derivative of cholic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bile acid, alcohol, or derivatives
- Oxosteroid
- 3-oxosteroid
- Cyclohexenone
- Cyclic ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052g-1559000000-0e39e44f77837ff493fb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-004i-1144900000-aaaa3f8e79735420065b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-d8ab6dc5d5eac735aeaa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0h00-0129000000-51216fd1229ea9215415 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2394000000-5b3df0250b69182f7fac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-0c82735c030bbf26a0ca | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gdi-1009000000-794ef9a9e00efb63545d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9005000000-ef861a1b9b9660eef48f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-5178d11479d0b8b77952 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0029000000-6c52d36b19e19581ecba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00mk-2093000000-547ddbf8eb4557ddc29b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0029000000-effe7eb9a96dc56f40e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-2394000000-2c3e5881abb59b8636c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-1930000000-f7b66c6aca3559dca1b3 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Takehara, Jun; Fujiwara, Naoya; Endou, Kyouko; Kawai, Junya; Hosokawa, Akemi; Sumitani, Naoko. Process for production of steroids. PCT Int. Appl. (2007), 138pp. |
|---|