| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:31:36 UTC |
|---|
| Update Date | 2020-05-11 20:55:01 UTC |
|---|
| BMDB ID | BMDB0000495 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Androstanediol |
|---|
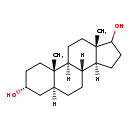
| Description | Androstanediol belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. Based on a literature review a significant number of articles have been published on Androstanediol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Androstane-3,17-diol | Kegg | | 3-a-Androstanediol | HMDB | | 3-alpha-Androstanediol | HMDB | | 3alpha-Androstanediol | HMDB | | 5 a-Androstane-3 a, 17 b-diol | HMDB | | 5 alpha-Androstane-3 alpha, 17 beta-diol | HMDB | | 5-a-Androstane-3-a, 17-b-diol | HMDB | | 5-alpha-Androstan-3-alpha,17-beta-diol | HMDB | | 5-alpha-Androstane-3-a, 17-beta-diol | HMDB | | 5-alpha-Androstane-3-alpha-17-beta-diol | HMDB | | 5 Androstane 3,17 diol | MeSH, HMDB | | 5 alpha Androstane 3 alpha,17 beta diol | MeSH, HMDB | | 5 alpha Androstane 3 beta,17 alpha diol | MeSH, HMDB | | 5 alpha Androstane 3 beta,17 beta diol | MeSH, HMDB | | 5 alpha Androstane 3alpha,17 beta diol | MeSH, HMDB | | 5 alpha-Androstane-3 alpha,17 beta-diol | MeSH, HMDB | | 5 alpha-Androstane-3 beta,17 alpha-diol | MeSH, HMDB | | 5 alpha-Androstane-3 beta,17 beta-diol | MeSH, HMDB | | 5 alpha-Androstane-3alpha,17 beta-diol | MeSH, HMDB | | 5 beta Androstane 3 alpha,17 beta diol | MeSH, HMDB | | 5 beta-Androstane-3 alpha,17 beta-diol | MeSH, HMDB | | 5-Androstane-3,17-diol | MeSH, HMDB | | 5alpha Androstane 3beta,17alpha diol | MeSH, HMDB | | 5alpha-Androstane-3beta,17alpha-diol | MeSH, HMDB | | Androstane 3,17 diol | MeSH, HMDB |
|
|---|
| Chemical Formula | C19H32O2 |
|---|
| Average Molecular Weight | 292.4562 |
|---|
| Monoisotopic Molecular Weight | 292.240230268 |
|---|
| IUPAC Name | (1S,2S,5R,7S,10R,11S,15S)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecane-5,14-diol |
|---|
| Traditional Name | (1S,2S,5R,7S,10R,11S,15S)-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecane-5,14-diol |
|---|
| CAS Registry Number | 25126-76-5 |
|---|
| SMILES | [H][C@@]12CCC(O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])C[C@H](O)CC[C@]12C |
|---|
| InChI Identifier | InChI=1S/C19H32O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12-17,20-21H,3-11H2,1-2H3/t12-,13+,14-,15-,16-,17?,18-,19-/m0/s1 |
|---|
| InChI Key | CBMYJHIOYJEBSB-UNPXRYTGSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as androgens and derivatives. These are 3-hydroxylated C19 steroid hormones. They are known to favor the development of masculine characteristics. They also show profound effects on scalp and body hair in humans. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Androstane steroids |
|---|
| Direct Parent | Androgens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Androgen-skeleton
- 17-hydroxysteroid
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- 3-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - C19 steroids (androgens) and derivatives (C07632 )
- Androstane and derivatives (C07632 )
|
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03gi-0290000000-ef7ce05726cbec3a35bd | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05fr-2237900000-35be1627cada18777db8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004l-0090000000-22d60c19836ea8b4c291 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0390000000-edb0fb77b27575b4b729 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00mk-2980000000-cd6eafa93f3164ed780a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-bc689c3cdfdf044c011e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-0090000000-bfbc140857bbdb62bcff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06vm-1290000000-bced1e06a8686f3501af | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0090000000-11452f48ce72555f1f28 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0090000000-11452f48ce72555f1f28 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000l-0090000000-ac9e93370094e8aebfa1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-0090000000-36f4d97265b0d994d628 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052b-3940000000-b87bad8b672cc5fb32b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aos-3900000000-0f8ce10c286f165d6e0f | View in MoNA |
|---|
|
|---|