| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:31:52 UTC |
|---|
| Update Date | 2020-04-22 15:03:55 UTC |
|---|
| BMDB ID | BMDB0000509 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Senecioic acid |
|---|
| Description | Senecioic acid, also known as 3-methylcrotonate or senecate, belongs to the class of organic compounds known as methyl-branched fatty acids. These are fatty acids with an acyl chain that has a methyl branch. Usually, they are saturated and contain only one or more methyl group. However, branches other than methyl may be present. Based on a literature review a significant number of articles have been published on Senecioic acid. |
|---|
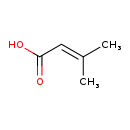
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,3-Dimethylacrylic acid | ChEBI | | 3-Methyl-2-butenoic acid | ChEBI | | 3-Methylcrotonic acid | ChEBI | | beta,beta-Dimethacrylic acid | ChEBI | | beta,beta-Dimethylacrylic acid | ChEBI | | beta-Methylcrotonic acid | ChEBI | | SENECIC ACID | ChEBI | | 3,3-Dimethylacrylate | Generator | | 3-Methyl-2-butenoate | Generator | | 3-Methylcrotonate | Generator | | b,b-Dimethacrylate | Generator | | b,b-Dimethacrylic acid | Generator | | beta,beta-Dimethacrylate | Generator | | Β,β-dimethacrylate | Generator | | Β,β-dimethacrylic acid | Generator | | b,b-Dimethylacrylate | Generator | | b,b-Dimethylacrylic acid | Generator | | beta,beta-Dimethylacrylate | Generator | | Β,β-dimethylacrylate | Generator | | Β,β-dimethylacrylic acid | Generator | | b-Methylcrotonate | Generator | | b-Methylcrotonic acid | Generator | | beta-Methylcrotonate | Generator | | Β-methylcrotonate | Generator | | Β-methylcrotonic acid | Generator | | SENECate | Generator | | Senecioate | Generator | | 3-Methyl-crotonate | HMDB | | 3-Methyl-crotonic acid | HMDB | | 3-Methylbut-2-enoate | HMDB | | 3-Methylbut-2-enoic acid | HMDB | | b,b-Dimethyl acrylate | HMDB | | b,b-Dimethyl acrylic acid | HMDB | | beta,beta-Dimethyl acrylate | HMDB | | Β,β-dimethyl acrylate | HMDB | | Β,β-dimethyl acrylic acid | HMDB | | Senecioic acid | ChEBI |
|
|---|
| Chemical Formula | C5H8O2 |
|---|
| Average Molecular Weight | 100.1158 |
|---|
| Monoisotopic Molecular Weight | 100.0524295 |
|---|
| IUPAC Name | 3-methylbut-2-enoic acid |
|---|
| Traditional Name | β,β-dimethacrylate |
|---|
| CAS Registry Number | 541-47-9 |
|---|
| SMILES | CC(C)=CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C5H8O2/c1-4(2)3-5(6)7/h3H,1-2H3,(H,6,7) |
|---|
| InChI Key | YYPNJNDODFVZLE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methyl-branched fatty acids. These are fatty acids with an acyl chain that has a methyl branch. Usually, they are saturated and contain only one or more methyl group. However, branches other than methyl may be present. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Methyl-branched fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methyl-branched fatty acid
- Unsaturated fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 65 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 10 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0zgl-9100000000-fad992c9622a048041c8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05fr-9200000000-47cf47043dcc621a0e70 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-0a4j-9000000000-92ace8b7ab3c75386bf2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-052b-9000000000-ade4eabc58077e6357c8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-0002-9000000000-b803e5f392f789d436dd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-9200000000-ec7954ac0255b8c3e03c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052u-9000000000-b6913d6ebb6c47b8e582 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052u-9000000000-fa9b01b0a4c8a64e570a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052b-9000000000-ad48b46e78b860433bb0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a5a-9000000000-b83dceb1bd0f86a7a491 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-9000000000-6b1083439ff18a99308e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9000000000-5289cc75fe7aae11de79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000t-9000000000-40f2a490f543e0322242 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9000000000-52adce5593fe6ee79db3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-053r-9000000000-2154212e0fc35a7a1e81 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-9000000000-7dd92e64eddf70e010a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000f-9000000000-87790895b0e53b400be7 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Iordache, Florin; Badica, Sonia; Ionescu, Alina; Badea, Florin. Synthesis of b,b-dimethylacrylic acid and its methyl and ethyl esters. Revistade Chimie (Bucharest, Romania) (1979), 30(7), 629-32. |
|---|