| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:31:57 UTC |
|---|
| Update Date | 2020-04-22 15:03:56 UTC |
|---|
| BMDB ID | BMDB0000513 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
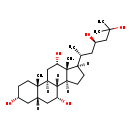
| Common Name | 5b-Cholestane-3a,7a,12a,23R,25-pentol |
|---|
| Description | 5b-Cholestane-3a,7a,12a,23R,25-pentol belongs to the class of organic compounds known as pentahydroxy bile acids, alcohols and derivatives. These are bile acids, alcohols or derivatives bearing five hydroxyl groups. Thus, 5b-cholestane-3a,7a,12a,23R,25-pentol is considered to be a sterol. Based on a literature review a small amount of articles have been published on 5b-Cholestane-3a,7a,12a,23R,25-pentol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 17-(3,5-Dihydroxy-1,5-dimethyl-hexyl)-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthrene-3,7,12-triol | HMDB |
|
|---|
| Chemical Formula | C27H48O5 |
|---|
| Average Molecular Weight | 452.667 |
|---|
| Monoisotopic Molecular Weight | 452.350174646 |
|---|
| IUPAC Name | (1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-14-[(2R,4R)-4,6-dihydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecane-5,9,16-triol |
|---|
| Traditional Name | (1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-14-[(2R,4R)-4,6-dihydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecane-5,9,16-triol |
|---|
| CAS Registry Number | 59906-14-8 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)C[C@@H](O)CC(C)(C)O |
|---|
| InChI Identifier | InChI=1S/C27H48O5/c1-15(10-18(29)14-25(2,3)32)19-6-7-20-24-21(13-23(31)27(19,20)5)26(4)9-8-17(28)11-16(26)12-22(24)30/h15-24,28-32H,6-14H2,1-5H3/t15-,16+,17-,18-,19-,20+,21+,22-,23+,24+,26+,27-/m1/s1 |
|---|
| InChI Key | OXSBBBPDYVCAKC-DYGXNTOZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pentahydroxy bile acids, alcohols and derivatives. These are bile acids, alcohols or derivatives bearing five hydroxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Pentahydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol
- Cholesterol-skeleton
- Pentahydroxy bile acid, alcohol, or derivatives
- Cholestane-skeleton
- 25-hydroxysteroid
- 23-hydroxysteroid
- 3-hydroxysteroid
- 7-hydroxysteroid
- 3-alpha-hydroxysteroid
- 12-hydroxysteroid
- Hydroxysteroid
- Tertiary alcohol
- Cyclic alcohol
- Secondary alcohol
- Polyol
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4r-3324900000-4eb492177cd3ff3b1d78 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0ue9-1620339000-8ab1e57d5c6fb0da7063 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0000900000-ef4a2790d00f39151d5b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-2005900000-a3c8abf978fcc74e3e28 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-07vi-4209700000-0530fc30e51b3d5bdd05 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ue9-0002900000-33ffd4c8b139bf760226 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0089-3004900000-c80999c8f40ac84967fb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9002000000-142d4259a51c5ae1ddec | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0409-0009700000-996ef26438e4bc39f389 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0670-4279500000-ec27c905a89e055a39d4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056r-9850000000-991e1aa56ec25a12a3fc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-31db0067a7126bac2473 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ugi-0003900000-68719f52b9a93bc9b349 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0003900000-c260caf34cf5aecd15cf | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Hoshita, Takahiko; Yasuhara, Misao; Kihira, Kenji; Kuramoto, Taiju. Comparative biochemical studies of bile acids and bile alcohols. V. Identification of (23S)-5a-cholestane-3a,7a,12a,23,25-pentol in cerebrotendinous xanthomatosis. Steroids (1976), 27(5), 657-64. |
|---|