| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:32:08 UTC |
|---|
| Update Date | 2020-04-21 18:15:25 UTC |
|---|
| BMDB ID | BMDB0000524 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 5b-Cholestane-3a,7a,12a,25-tetrol |
|---|
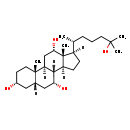
| Description | 5beta-Cholestane-3alpha,7alpha,12alpha,25-tetrol, also known as cholestane-3,7,12,25-tetrol or 3α,7α,12α,25-tetrahydroxycoprostane, belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. Thus, 5beta-cholestane-3alpha,7alpha,12alpha,25-tetrol is considered to be a bile acid. Based on a literature review a significant number of articles have been published on 5beta-Cholestane-3alpha,7alpha,12alpha,25-tetrol. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5b-Cholestane-3a,7a,12a,25-tetrol | Generator | | 5Β-cholestane-3α,7α,12α,25-tetrol | Generator | | 5 beta Cholestane-3 alpha,7 alpha,12 alpha,25-tetrol | HMDB | | 5 beta-Cholestane-3 beta,7 alpha,12 alpha,25-tetrol | HMDB | | Cholestane-3,7,12,25-tetrol | HMDB | | (3Α,5β,7α,12α)-cholestane-3,7,12,25-tetrol | HMDB | | 3Α,7α,12α,25-tetrahydroxycoprostane | HMDB | | (3alpha,5beta,7alpha,12alpha)-Cholestane-3,7,12,25-tetrol | HMDB | | 3alpha,7alpha,12alpha,25-Tetrahydroxycoprostane | HMDB | | 5b-Cholestan-3a,7a,12a,25-tetrol | HMDB | | 5beta-Cholestane-3alpha,7alpha,12alpha,25-tetrol | Generator |
|
|---|
| Chemical Formula | C27H48O4 |
|---|
| Average Molecular Weight | 436.677 |
|---|
| Monoisotopic Molecular Weight | 436.355260026 |
|---|
| IUPAC Name | (1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-14-[(2R)-6-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecane-5,9,16-triol |
|---|
| Traditional Name | (1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-14-[(2R)-6-hydroxy-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecane-5,9,16-triol |
|---|
| CAS Registry Number | 18866-87-0 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])C[C@H](O)[C@]12C)[C@H](C)CCCC(C)(C)O |
|---|
| InChI Identifier | InChI=1S/C27H48O4/c1-16(7-6-11-25(2,3)31)19-8-9-20-24-21(15-23(30)27(19,20)5)26(4)12-10-18(28)13-17(26)14-22(24)29/h16-24,28-31H,6-15H2,1-5H3/t16-,17+,18-,19-,20+,21+,22-,23+,24+,26+,27-/m1/s1 |
|---|
| InChI Key | NTIXPPFPXLYJCT-RNUSRIHUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol
- Cholesterol-skeleton
- 25-hydroxysteroid
- 3-hydroxysteroid
- 12-hydroxysteroid
- 7-hydroxysteroid
- Hydroxysteroid
- 3-alpha-hydroxysteroid
- Cyclic alcohol
- Tertiary alcohol
- Secondary alcohol
- Polyol
- Organic oxygen compound
- Alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | Not Available |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_4_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0000900000-ce7599af15d682b569c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-1005900000-372cbf89a7b70f683e60 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f7a-2209200000-de49d287713beec95040 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kr-0001900000-f8627b8fe61026d323ea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014r-0002900000-e0dbc20b7cbe891c1ef9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uy0-2017900000-ec2da6f1ebd80a8be486 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0000900000-3776cbb3f067b3114cea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uy3-9431700000-e8fb6d533c341214e749 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0btc-9531000000-a56819513601843f6933 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000900000-0c413525e4be8a74c95d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0000900000-ac053244f41b05618d02 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0001900000-46fbc2ed078e9f536fdc | View in MoNA |
|---|
|
|---|