| Synonyms | | Value | Source |
|---|
| 5a-Tetrahydrocortisol | Generator | | 5Α-tetrahydrocortisol | Generator | | 3,11,17,21-Tetrahydroxypregnan-20-one | HMDB | | 3a,11b,17,21-Tetrahydroxy-5a-pregnan-20-one | HMDB | | 3a,11b,17a,21-Tetrahydroxy-5a-pregnan-20-one | HMDB | | 3a,5a-Tetrahydrocortisol | HMDB | | 3a-Allotetrahydrocortisol | HMDB | | 3alpha, 5alpha-Tetrahydrocortisol | HMDB | | 3alpha,5alpha-Tetrahydrocortisol | HMDB | | 5a-Pregnane-3a,11b,17a,21-tetraol-20-one | HMDB | | 5a-Pregnane-3a,11b,17a,21-tetrol-20-one | HMDB | | a-THF | HMDB | | Allo-3a-tetrahydrocortisol | HMDB | | Allo-3alpha-tetrahydrocortisol | HMDB | | Allo-tetrahydrocortisol | HMDB | | Allopregnane-3a,11b,17a,21-tetrol-20-one | HMDB | | Allopregnane-3a,11b,21-tetrol-20-one | HMDB | | Allopregnane-3alpha,11beta,17alpha,21-tetrol-20-one | HMDB | | Allotetrahydro-compound F | HMDB | | Allotetrahydro-cortisol | HMDB | | Allotetrahydrocompound F | HMDB | | Allotetrahydrocortisol | HMDB | | alpha-THF | HMDB | | ATHF | HMDB | | Kendall'S compound c | HMDB | | Reichstein'S substance C | HMDB | | Tetrahydro-allocortisol | HMDB | | Tetrahydroallocortisol | HMDB | | Wintersteiner'S compound D | HMDB | | Allotetrahydrocortisol, (3beta,5alpha,11beta)-isomer | HMDB | | 5AlphaTHF | HMDB | | Allotetrahydrocortisol, (3alpha,5beta,11alpha)-isomer | HMDB | | Allotetrahydrocortisol, (5alpha,11beta)-isomer | HMDB | | Allotetrahydrocortisol, (3beta,5beta,11beta)-isomer | HMDB | | (3alpha,5alpha,11beta)-3,11,17,21-Tetrahydroxypregnan-20-one | HMDB | | (3Α,5α,11β)-3,11,17,21-tetrahydroxypregnan-20-one | HMDB | | 3alpha,11beta,17,21-Tetrahydroxy-5alpha-pregnan-20-one | HMDB | | 3alpha,11beta,17alpha,21-Tetrahydroxy-5alpha-pregnan-20-one | HMDB | | 3Α,11β,17,21-tetrahydroxy-5α-pregnan-20-one | HMDB | | 3Α,11β,17α,21-tetrahydroxy-5α-pregnan-20-one | HMDB | | 3Α,5α-tetrahydrocortisol | HMDB | | 5alpha-Pregnane-3alpha,11beta,17alpha,21-tetraol-20-one | HMDB | | 5alpha-Pregnane-3alpha,11beta,17alpha,21-tetrol-20-one | HMDB | | 5alpha-THF | HMDB | | 5Α-pregnane-3α,11β,17α,21-tetraol-20-one | HMDB | | 5Α-pregnane-3α,11β,17α,21-tetrol-20-one | HMDB | | 5Α-THF | HMDB | | Allopregnane-3α,11β,17α,21-tetrol-20-one | HMDB | | Allo-3α-tetrahydrocortisol | HMDB | | Α-THF | HMDB | | 5alpha-Tetrahydrocortisol | HMDB |
|
|---|
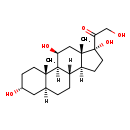
| IUPAC Name | 2-hydroxy-1-[(1S,2S,5R,7S,10S,11S,14R,15S,17S)-5,14,17-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]ethan-1-one |
|---|
| Traditional Name | 2-hydroxy-1-[(1S,2S,5R,7S,10S,11S,14R,15S,17S)-5,14,17-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]ethanone |
|---|
| InChI Identifier | InChI=1S/C21H34O5/c1-19-7-5-13(23)9-12(19)3-4-14-15-6-8-21(26,17(25)11-22)20(15,2)10-16(24)18(14)19/h12-16,18,22-24,26H,3-11H2,1-2H3/t12-,13+,14-,15-,16-,18+,19-,20-,21-/m0/s1 |
|---|