| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:32:48 UTC |
|---|
| Update Date | 2020-04-22 15:04:11 UTC |
|---|
| BMDB ID | BMDB0000559 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 3-Methoxy-4-hydroxyphenylethyleneglycol sulfate |
|---|
| Description | 3-Methoxy-4-hydroxyphenylethyleneglycol sulfate, also known as mopeg sulfate or [4-(1,2-dihydroxyethyl)-2-methoxyphenyl]oxidanesulfonate, belongs to the class of organic compounds known as phenylsulfates. Phenylsulfates are compounds containing a sulfuric acid group conjugated to a phenyl group. Based on a literature review very few articles have been published on 3-Methoxy-4-hydroxyphenylethyleneglycol sulfate. |
|---|
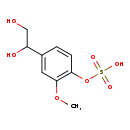
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Methoxy-4-hydroxyphenylethyleneglycol sulfuric acid | Generator | | 3-Methoxy-4-hydroxyphenylethyleneglycol sulphate | Generator | | 3-Methoxy-4-hydroxyphenylethyleneglycol sulphuric acid | Generator | | (3-Methoxy-4-hydroxyphenyl)ethylene glycol sulfate | HMDB | | (3-Methoxy-4-hydroxyphenyl)ethylene glycol sulphate | HMDB | | (3-Methoxy-4-hydroxyphenyl)glycol O-sulfate | HMDB | | (3-Methoxy-4-hydroxyphenyl)glycol O-sulphate | HMDB | | (3-Methoxy-4-hydroxyphenyl)glycol sulfate ester | HMDB | | (3-Methoxy-4-hydroxyphenyl)glycol sulphate ester | HMDB | | 3-Methoxy-4-hydroxyphenylglycol 4-sulfate | HMDB | | 3-Methoxy-4-hydroxyphenylglycol 4-sulphate | HMDB | | MOPEG sulfate | HMDB | | MOPEG sulphate | HMDB | | [4-(1,2-Dihydroxyethyl)-2-methoxyphenyl]oxidanesulfonate | HMDB | | [4-(1,2-Dihydroxyethyl)-2-methoxyphenyl]oxidanesulphonate | HMDB | | [4-(1,2-Dihydroxyethyl)-2-methoxyphenyl]oxidanesulphonic acid | HMDB | | MOPEG sulfuric acid | HMDB | | MOPEG sulphuric acid | HMDB | | 1-[3-Methoxy-4-(sulfooxy)phenyl]-1,2-ethanediol | HMDB | | 4-Hydroxy-3-methoxyphenylglycol sulfate | HMDB | | HMPG Sulfate | HMDB | | MHPG-SO4 | HMDB |
|
|---|
| Chemical Formula | C9H12O7S |
|---|
| Average Molecular Weight | 264.252 |
|---|
| Monoisotopic Molecular Weight | 264.030373428 |
|---|
| IUPAC Name | [4-(1,2-dihydroxyethyl)-2-methoxyphenyl]oxidanesulfonic acid |
|---|
| Traditional Name | mopeg sulfate |
|---|
| CAS Registry Number | 3415-67-6 |
|---|
| SMILES | COC1=C(OS(O)(=O)=O)C=CC(=C1)C(O)CO |
|---|
| InChI Identifier | InChI=1S/C9H12O7S/c1-15-9-4-6(7(11)5-10)2-3-8(9)16-17(12,13)14/h2-4,7,10-11H,5H2,1H3,(H,12,13,14) |
|---|
| InChI Key | WUFPNASKMLJSND-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylsulfates. Phenylsulfates are compounds containing a sulfuric acid group conjugated to a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic sulfuric acids and derivatives |
|---|
| Sub Class | Arylsulfates |
|---|
| Direct Parent | Phenylsulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylsulfate
- Phenoxy compound
- Anisole
- Phenol ether
- Methoxybenzene
- Alkyl aryl ether
- Monocyclic benzene moiety
- Benzenoid
- Sulfuric acid monoester
- Sulfate-ester
- Sulfuric acid ester
- 1,2-diol
- Secondary alcohol
- Ether
- Primary alcohol
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Aromatic alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f89-2790000000-7c3903505d875c849801 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0200-4129000000-7ccee38f4fc39858f4d6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-d64015fcc7dd0dfcf7c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-0890000000-e8d58d2012b8901d1ab8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udr-7930000000-be29f6411b0949ccca7f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-9608dad0e094a41af4c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uyi-1960000000-b564e86015565735c1dc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00yi-3900000000-0ea9a64da4cfad1e32a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-38ce94dae333569cc61d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-2090000000-91698b2a2ae987fff98a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052b-9100000000-9857c45f245bf465a5d1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014j-0090000000-7f17610c197b80fd1fe3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-016r-0900000000-25e5b26f8f0170dd363b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-1900000000-00717c244fd4e704eb9f | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|