| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:33:18 UTC |
|---|
| Update Date | 2020-05-11 20:37:15 UTC |
|---|
| BMDB ID | BMDB0000594 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Glutamylphenylalanine |
|---|
| Description | gamma-Glutamylphenylalanine, also known as L-gamma-glu-L-phe or N-(γ-L-glutamyl)phenylalanine, belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Based on a literature review a significant number of articles have been published on gamma-Glutamylphenylalanine. |
|---|
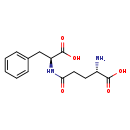
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-2-Amino-4-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}butanoic acid | ChEBI | | L-gamma-Glu-L-phe | ChEBI | | L-gamma-Glutamyl-L-phenylalanine | ChEBI | | N-(gamma-L-Glutamyl)phenylalanine | ChEBI | | N-L-gamma-Glutamyl-3-phenyl-L-alanine | ChEBI | | N-L-gamma-Glutamyl-L-phenylalanine | ChEBI | | (2S)-2-Amino-4-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}butanoate | Generator | | L-g-Glu-L-phe | Generator | | L-Γ-glu-L-phe | Generator | | L-g-Glutamyl-L-phenylalanine | Generator | | L-Γ-glutamyl-L-phenylalanine | Generator | | N-(g-L-Glutamyl)phenylalanine | Generator | | N-(Γ-L-glutamyl)phenylalanine | Generator | | N-L-g-Glutamyl-3-phenyl-L-alanine | Generator | | N-L-Γ-glutamyl-3-phenyl-L-alanine | Generator | | N-L-g-Glutamyl-L-phenylalanine | Generator | | N-L-Γ-glutamyl-L-phenylalanine | Generator | | g-Glutamylphenylalanine | Generator | | Γ-glutamylphenylalanine | Generator | | gamma-Glu-phe | MeSH | | γ-Glu-Phe | HMDB, Generator | | γ-L-Glu-L-Phe | HMDB | | γ-L-Glutamyl-L-phenylalanine | HMDB | | N-γ-Glutamylphenylalanine | HMDB | | N-L-γ-Glutamylphenylalanine | HMDB | | gamma-L-Glu-L-Phe | HMDB | | gamma-L-Glutamyl-L-phenylalanine | HMDB | | N-gamma-Glutamylphenylalanine | HMDB | | N-L-gamma-Glutamylphenylalanine | HMDB | | H-γ-Glu-Phe-OH | HMDB | | H-gamma-Glu-Phe-OH | HMDB | | gamma-Glutamylphenylalanine | HMDB, ChEBI | | N-gamma-L-Glutamyl-L-phenylalanine | HMDB | | N-γ-L-Glutamyl-L-phenylalanine | HMDB | | g-Glu-Phe | Generator, HMDB |
|
|---|
| Chemical Formula | C14H18N2O5 |
|---|
| Average Molecular Weight | 294.3031 |
|---|
| Monoisotopic Molecular Weight | 294.121571696 |
|---|
| IUPAC Name | (2S)-2-amino-4-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}butanoic acid |
|---|
| Traditional Name | gamma-glutamylphenylalanine |
|---|
| CAS Registry Number | 7432-24-8 |
|---|
| SMILES | N[C@@H](CCC(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C14H18N2O5/c15-10(13(18)19)6-7-12(17)16-11(14(20)21)8-9-4-2-1-3-5-9/h1-5,10-11H,6-8,15H2,(H,16,17)(H,18,19)(H,20,21)/t10-,11-/m0/s1 |
|---|
| InChI Key | XHHOHZPNYFQJKL-QWRGUYRKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Phenylalanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylalanine or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- 3-phenylpropanoic-acid
- Alpha-amino acid
- Amphetamine or derivatives
- L-alpha-amino acid
- Monocyclic benzene moiety
- Benzenoid
- Dicarboxylic acid or derivatives
- Amino acid
- Carboximidic acid
- Carboximidic acid derivative
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid
- Organic nitrogen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organooxygen compound
- Primary amine
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9350000000-cee136a59eda9bbc48b1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00fu-9633100000-04cb3c5e9af1918fedf0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT , positive | splash10-014i-0900000000-3c15b0ec8b0aa7b4f562 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ot-1490000000-8a194f7de818cb11e47b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03xs-2960000000-8471d66cf80c273476e9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9200000000-cee53e3b344fef2f519e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0290000000-f75848c2ae5c1246f8d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0035-1790000000-fd452e6dee821306584b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02fx-9500000000-9ba84b5fd475503d8cd7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01tc-0980000000-3d8a781a1b7c5419cc5c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0imm-2930000000-ee52208f821d132b59a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006x-9800000000-49bcc3238b9451889ddf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-1790000000-683c9d45013f9557d41c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-2910000000-d284cbb0231807781a10 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ac0-9400000000-939d577cffe7458ad422 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|