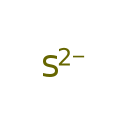| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:33:21 UTC |
|---|
| Update Date | 2020-06-04 21:54:15 UTC |
|---|
| BMDB ID | BMDB0000598 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Sulfide |
|---|
| Description | Sulfide, also known as sulphide or S(2-), belongs to the class of inorganic compounds known as other non-metal sulfides. These are inorganic compounds containing a sulfur atom of an oxidation state of -2, in which the heaviest atom bonded to the oxygen belongs to the class of other non-metals. Sulfide exists as a solid, possibly soluble (in water), and possibly neutral molecule. Sulfide exists in all living species, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| S(2-) | ChEBI | | Sulphide | ChEBI | | Sulfanediide | HMDB | | Sulfur | HMDB | | Sulphide(2-) | HMDB | | Sulfide | ChEBI |
|
|---|
| Chemical Formula | S |
|---|
| Average Molecular Weight | 32.065 |
|---|
| Monoisotopic Molecular Weight | 31.97207069 |
|---|
| IUPAC Name | sulfanediide |
|---|
| Traditional Name | sulfanediide |
|---|
| CAS Registry Number | 18496-25-8 |
|---|
| SMILES | [S--] |
|---|
| InChI Identifier | InChI=1S/S/q-2 |
|---|
| InChI Key | UCKMPCXJQFINFW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of inorganic compounds known as other non-metal sulfides. These are inorganic compounds containing a sulfur atom of an oxidation state of -2, in which the heaviest atom bonded to the oxygen belongs to the class of other non-metals. |
|---|
| Kingdom | Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
| Class | Other non-metal organides |
|---|
| Sub Class | Other non-metal sulfides |
|---|
| Direct Parent | Other non-metal sulfides |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 112 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | |
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Biospecimen Locations | - Brain
- Cartilage
- Epidermis
- Hair
- Liver
- Milk
- Platelet
|
|---|
| Pathways | |
|---|
| Normal Concentrations |
|---|
| |
| Brain | Expected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Cartilage | Expected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Epidermis | Expected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Hair | Expected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Liver | Expected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Milk | Detected and Quantified | 9979.729 uM | Not Specified | Not Specified | Normal | | details | | Platelet | Expected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details |
|
|---|
| Abnormal Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| HMDB ID | HMDB0000598 |
|---|
| DrugBank ID | Not Available |
|---|
| Phenol Explorer Compound ID | Not Available |
|---|
| FooDB ID | FDB022136 |
|---|
| KNApSAcK ID | Not Available |
|---|
| Chemspider ID | 27079 |
|---|
| KEGG Compound ID | C00087 |
|---|
| BioCyc ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| Wikipedia Link | Sulfide |
|---|
| METLIN ID | Not Available |
|---|
| PubChem Compound | 29109 |
|---|
| PDB ID | Not Available |
|---|
| ChEBI ID | 15138 |
|---|
| References |
|---|
| Synthesis Reference | Green, Martina; Verkoczy, Bela; Lown, Elizabeth M.; Strausz, Otto P. The reactions of sulfur atoms with propadiene and 1,2-butadiene. Canadian Journal of Chemistry (1985), 63(3), 667-75. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| General References | - Park, Y. W; Juárez, Manuela ; Ramos, M.; Haenlein, G. F. W. (2007). Park, Y. W; Juárez, Manuela ; Ramos, M.; Haenlein, G. F. W.. Physico-chemical characteristics of goat and sheep milk. Small Ruminant Res.(2007) 68:88-113 doi: 10.1016/j.smallrumres.2006.09.013. Small Ruminant Research.
|
|---|