| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:33:32 UTC |
|---|
| Update Date | 2020-04-22 15:04:23 UTC |
|---|
| BMDB ID | BMDB0000609 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
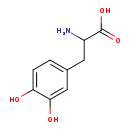
| Common Name | DL-Dopa |
|---|
| Description | DL-DOPA, also known as (+-)-dopa or (R,S)-dopa, belongs to the class of organic compounds known as tyrosine and derivatives. Tyrosine and derivatives are compounds containing tyrosine or a derivative thereof resulting from reaction of tyrosine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Based on a literature review a significant number of articles have been published on DL-DOPA. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+-)-3-(3,4-Dihydroxyphenyl)alanine | ChEBI | | (+-)-Dopa | ChEBI | | (R,S)-Dopa | ChEBI | | 3',4'-Dihydroxyphenylalanine | ChEBI | | 3-Hydroxy-DL-tyrosine | ChEBI | | 3-Hydroxytyrosine | ChEBI | | beta-(3,4-Dihydroxyphenyl)-DL-alpha-alanine | ChEBI | | DL-3,4-Dopa | ChEBI | | DL-beta-(3,4-Dihydroxyphenyl)-alpha-alanine | ChEBI | | DL-beta-(3,4-Dihydroxyphenyl)alanine | ChEBI | | DL-Dihydroxyphenylalanine | ChEBI | | DL-Dioxyphenylalanine | ChEBI | | b-(3,4-Dihydroxyphenyl)-DL-a-alanine | Generator | | Β-(3,4-dihydroxyphenyl)-DL-α-alanine | Generator | | DL-b-(3,4-Dihydroxyphenyl)-a-alanine | Generator | | DL-Β-(3,4-dihydroxyphenyl)-α-alanine | Generator | | DL-b-(3,4-Dihydroxyphenyl)alanine | Generator | | DL-Β-(3,4-dihydroxyphenyl)alanine | Generator | | (+/-) 3-(3,4-dihydroxyphenyl)alanine | HMDB | | 2-amino-3-(3,4-Dihydroxyphenyl)propanoate | HMDB | | 2-amino-3-(3,4-Dihydroxyphenyl)propanoic acid | HMDB | | 3,4-Dihydroxy-DL-phenylalanine | HMDB | | 3,4-Dihydroxyphenylalanine | HMDB, MeSH | | 3-(3,4-Dihydroxyphenyl)-DL-alanine | HMDB | | a-amino-3,4-Dihydroxy-benzenepropanoate | HMDB | | a-amino-3,4-Dihydroxy-benzenepropanoic acid | HMDB | | alpha-amino-3,4-Dihydroxy-benzenepropanoate | HMDB | | alpha-amino-3,4-Dihydroxy-benzenepropanoic acid | HMDB | | alpha-amino-Hydrocaffeic acid | HMDB | | b-(3,4-Dihydroxyphenyl)-a-alanine | HMDB | | beta-(3,4-Dihydroxyphenyl)-alpha-alanine | HMDB | | DL-3',4'-Dihydroxyphenylalanine | HMDB | | DL-3,4-Dihydroxyphenylalanine | HMDB | | DL-3-Hydroxytyrosine | HMDB | | DL-4,5-Dihydroxyphenylalanine | HMDB | | Dopa | MeSH, HMDB | | 3 Hydroxy DL tyrosine | MeSH, HMDB | | 3,4 Dihydroxyphenylalanine | MeSH, HMDB | | beta-Hydroxytyrosine | MeSH, HMDB | | Dihydroxyphenylalanine hydrochloride, (2:1) | MeSH, HMDB | | beta Hydroxytyrosine | MeSH, HMDB | | Dihydroxyphenylalanine | MeSH, HMDB |
|
|---|
| Chemical Formula | C9H11NO4 |
|---|
| Average Molecular Weight | 197.1879 |
|---|
| Monoisotopic Molecular Weight | 197.068807845 |
|---|
| IUPAC Name | 2-amino-3-(3,4-dihydroxyphenyl)propanoic acid |
|---|
| Traditional Name | 3',4'-dihydroxyphenylalanine |
|---|
| CAS Registry Number | 63-84-3 |
|---|
| SMILES | NC(CC1=CC=C(O)C(O)=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C9H11NO4/c10-6(9(13)14)3-5-1-2-7(11)8(12)4-5/h1-2,4,6,11-12H,3,10H2,(H,13,14) |
|---|
| InChI Key | WTDRDQBEARUVNC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tyrosine and derivatives. Tyrosine and derivatives are compounds containing tyrosine or a derivative thereof resulting from reaction of tyrosine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Tyrosine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tyrosine or derivatives
- Phenylalanine or derivatives
- 3-phenylpropanoic-acid
- Amphetamine or derivatives
- Alpha-amino acid
- Catechol
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Phenol
- Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- Amino acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Amine
- Primary amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 270 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 3.6 mg/mL | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-3900000000-e27bccc5fb602b6f1098 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0002-2391000000-1855886013a2095957cc | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0ue9-0900000000-9a2dc91dda37ccd6d764 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0a59-3900000000-51fb8377ac819b0b3100 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00or-9200000000-467c6d2d6b1c5185bac8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-000b-0900000000-0805d147ed6a4a5615f5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-000i-0900000000-0535cbfb1f2866998bd8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-0a59-0900000000-02a7e481355ecaedcf9f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , negative | splash10-000i-0900000000-664479c3e56237ecf94c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0udr-0900000000-431d201400cf2fb08fad | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-052r-0900000000-9177ea4b8f53bd44dc93 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0a4i-0900000000-bf5afb57c3944eb31c73 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0udr-0900000000-791d6239bfe8cf2cb189 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-0900000000-02a7e481355ecaedcf9f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-9de24eed142a3f0928f1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-000b-0900000000-412eb3979672d859993d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0900000000-2a1dc85195d73c3a878b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udr-0900000000-02b57b8211288da98141 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0900000000-0f14fd981694b50996ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uea-0900000000-7cd68d059960262e024c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-31563ea2d36808cf260c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-8900000000-07fa521201594d25db7d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-079ea72e0cb84251b6c5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0092-1900000000-6bcbd38a361ba2abf5bd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9800000000-6e59379b40a21e5e2a33 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-65da392a07028d302f21 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0k9i-0900000000-ec3a1ce52a52091b7d94 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-00di-4900000000-51469f201a754d9d871d | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|