| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:34:10 UTC |
|---|
| Update Date | 2020-05-11 20:21:15 UTC |
|---|
| BMDB ID | BMDB0000643 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Coproporphyrin I |
|---|
| Description | Coproporphyrin I belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. Coproporphyrin I is possibly soluble (in water) and a very strong basic compound (based on its pKa). Coproporphyrin I exists in all eukaryotes, ranging from yeast to humans. In cattle, coproporphyrin i is involved in the metabolic pathway called the porphyrin metabolism pathway. Coproporphyrin I is a potentially toxic compound. |
|---|
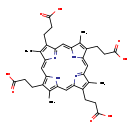
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,8,13,17-Tetramethylporphyrin-2,7,12,18-tetrapropanoic acid | ChEBI | | 3,8,13,17-Tetramethylporphyrin-2,7,12,18-tetrapropanoate | Generator | | 3,8,13,18-Tetramethyl-2,7,12,17-porphinetetrapropionate | HMDB | | 3,8,13,18-Tetramethyl-2,7,12,17-porphinetetrapropionic acid | HMDB | | 3,8,13,18-Tetramethyl-21H,23H-porphine-2,7,12,17-tetrapropionate | HMDB | | 3,8,13,18-Tetramethyl-21H,23H-porphine-2,7,12,17-tetrapropionic acid | HMDB |
|
|---|
| Chemical Formula | C36H38N4O8 |
|---|
| Average Molecular Weight | 654.7089 |
|---|
| Monoisotopic Molecular Weight | 654.268964212 |
|---|
| IUPAC Name | 3-[9,14,19-tris(2-carboxyethyl)-5,10,15,20-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1(20),2,4,6(24),7,9,11,13(22),14,16,18-undecaen-4-yl]propanoic acid |
|---|
| Traditional Name | 3-[9,14,19-tris(2-carboxyethyl)-5,10,15,20-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1(20),2,4,6(24),7,9,11,13(22),14,16,18-undecaen-4-yl]propanoic acid |
|---|
| CAS Registry Number | 531-14-6 |
|---|
| SMILES | CC1=C(CCC(O)=O)/C2=C/C3=N/C(=C\C4=C(C)C(CCC(O)=O)=C(N4)/C=C4\N=C(\C=C\1/N\2)C(CCC(O)=O)=C4C)/C(CCC(O)=O)=C3C |
|---|
| InChI Identifier | InChI=1S/C36H38N4O8/c1-17-21(5-9-33(41)42)29-14-26-19(3)23(7-11-35(45)46)31(39-26)16-28-20(4)24(8-12-36(47)48)32(40-28)15-27-18(2)22(6-10-34(43)44)30(38-27)13-25(17)37-29/h13-16,37,40H,5-12H2,1-4H3,(H,41,42)(H,43,44)(H,45,46)(H,47,48)/b25-13-,26-14-,27-15-,28-16-,29-14-,30-13-,31-16-,32-15- |
|---|
| InChI Key | VORBHEGMEBOMMB-JRHDEHKPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052g-2000069000-b0400ff816c8bb479aa9 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_3_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0000039000-f3b4207c1c84e6625cb5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4u-0000097000-25bf85caccefc18e4e75 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ot-1000090000-f4fcb2a24b23cd617b5c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000009000-9d1a49bb4b1cf969e8e6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zg0-1000039000-f24da7ff41769ba0e5be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000042000-ef49befaf8862d948c2b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udu-0000029000-2d152ebc7ba864c16d1d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0000092000-7658c361acf72da2ec31 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0000090000-54eff7e29e4dd69eaaa8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05n0-0000039000-65aba1df7d5d3a27e3de | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05mx-0000095000-745ccb4c5e74570921be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-0000091000-a506f665cd46b8d0f47d | View in MoNA |
|---|
|
|---|