Showing metabocard for Indoleacrylic acid (BMDB0000734)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-09-30 22:35:42 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2020-04-22 15:05:03 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMDB ID | BMDB0000734 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Indoleacrylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Indoleacrylic acid, also known as 2-indoleacrylate, belongs to the class of organic compounds known as indoles. Indoles are compounds containing an indole moiety, which consists of pyrrole ring fused to benzene to form 2,3-benzopyrrole. Indoleacrylic acid is a weakly acidic compound (based on its pKa). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
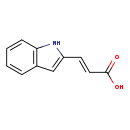
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C11H9NO2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight | 187.1947 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight | 187.063328537 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | (2E)-3-(1H-indol-2-yl)prop-2-enoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | (2E)-3-(1H-indol-2-yl)prop-2-enoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 1204-06-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | OC(=O)\C=C\C1=CC2=C(N1)C=CC=C2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C11H9NO2/c13-11(14)6-5-9-7-8-3-1-2-4-10(8)12-9/h1-7,12H,(H,13,14)/b6-5+ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | SXOUIMVOMIGLHO-AATRIKPKSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as indoles. Indoles are compounds containing an indole moiety, which consists of pyrrole ring fused to benzene to form 2,3-benzopyrrole. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Indoles and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Indoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Indoles | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ontology | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Expected but not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofunction | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Application | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biospecimen Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB0000734 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer Compound ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | FDB000938 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | C00000111 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | 10607876 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Compound ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | 5702 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound | 15030923 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 90333 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Moffatt, J. S. Preparation of b-3-indolylacrylic acid. Journal of the Chemical Society (1957), 1442-3.; Bauguess, Lyle C.; Berg, Clarence P. The availability of indole derivatives for supplementing diets deficient in tryptophan. Proceedings of the Iowa Academy of Science (1933), 40 110-11. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||