| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:35:45 UTC |
|---|
| Update Date | 2020-05-11 20:21:28 UTC |
|---|
| BMDB ID | BMDB0000737 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
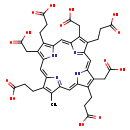
| Common Name | Heptacarboxylporphyrin I |
|---|
| Description | Heptacarboxylporphyrin I, also known as heptaporphyrin, belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. Heptacarboxylporphyrin I, with regard to humans, has been found to be associated with several diseases such as porphyria cutanea tarda, liver disease, acute intermittent porphyria, and variegate porphyria; heptacarboxylporphyrin I has also been linked to the inborn metabolic disorder hereditary coproporphyria. Based on a literature review a small amount of articles have been published on Heptacarboxylporphyrin I. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Heptacarboxylic acid porphyrin I | HMDB | | Heptaporphyrin | HMDB | | 3-[9,14,19-Tris(2-carboxyethyl)-10,15,20-tris(carboxymethyl)-5-methyl-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3(24),4,6,8,10,12,14,16(22),17,19-undecaen-4-yl]propanoate | HMDB |
|
|---|
| Chemical Formula | C39H38N4O14 |
|---|
| Average Molecular Weight | 786.7374 |
|---|
| Monoisotopic Molecular Weight | 786.238451944 |
|---|
| IUPAC Name | 3-[9,14,19-tris(2-carboxyethyl)-10,15,20-tris(carboxymethyl)-5-methyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1(20),2,4,6(24),7,9,11,13(22),14,16,18-undecaen-4-yl]propanoic acid |
|---|
| Traditional Name | 3-[9,14,19-tris(2-carboxyethyl)-10,15,20-tris(carboxymethyl)-5-methyl-21,22,23,24-tetraazapentacyclo[16.2.1.1^{3,6}.1^{8,11}.1^{13,16}]tetracosa-1(20),2,4,6(24),7,9,11,13(22),14,16,18-undecaen-4-yl]propanoic acid |
|---|
| CAS Registry Number | 65406-45-3 |
|---|
| SMILES | CC1=C(CCC(O)=O)\C2=C\C3=C(CC(O)=O)C(CCC(O)=O)=C(N3)\C=C3/N=C(/C=C4\N\C(=C/C1=N2)C(CCC(O)=O)=C4CC(O)=O)C(CCC(O)=O)=C3CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C39H38N4O14/c1-17-18(2-6-33(44)45)26-14-30-23(11-38(54)55)20(4-8-35(48)49)28(42-30)16-32-24(12-39(56)57)21(5-9-36(50)51)29(43-32)15-31-22(10-37(52)53)19(3-7-34(46)47)27(41-31)13-25(17)40-26/h13-16,41-42H,2-12H2,1H3,(H,44,45)(H,46,47)(H,48,49)(H,50,51)(H,52,53)(H,54,55)(H,56,57)/b25-13-,26-14-,27-13-,28-16-,29-15-,30-14-,31-15-,32-16- |
|---|
| InChI Key | GWTVAIDNCPVMLP-YBWGHNILSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 208 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-106r-0000000900-1a5659367722a3b91d07 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-0000002900-85dfdd282818a628f93a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-0000009700-49b4b052511c4ae6e5ab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00xu-0000000900-ab85973f83598078c240 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0000001900-1a9755eb8354eff16f28 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-2000000900-453451d4dc4aab7fd692 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00xs-0000002900-7fb9688f9c1ee33b1308 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00aj-0000009600-c6f4c536d4eb7fbe2584 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003s-0000009500-95d5d3990319dc7e8707 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dj-0000006900-3a85eeb4b06df50b7894 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006t-0000009700-fa3a78fcf7166dc4eeb0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kb-0000009100-6f56ce92b761bddef4bd | View in MoNA |
|---|
|
|---|