| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:36:23 UTC |
|---|
| Update Date | 2020-05-11 20:55:10 UTC |
|---|
| BMDB ID | BMDB0000774 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
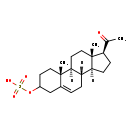
| Common Name | Pregnenolone sulfate |
|---|
| Description | Pregnenolone sulfate belongs to the class of organic compounds known as sulfated steroids. These are sterol lipids containing a sulfate group attached to the steroid skeleton. Based on a literature review a significant number of articles have been published on Pregnenolone sulfate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Pregnenolone sulfuric acid | Generator | | Pregnenolone sulphate | Generator | | Pregnenolone sulphuric acid | Generator | | 5-Pregnen-3b-sulfate-20-one | HMDB | | 5-Pregnen-3b-sulphate-20-one | HMDB | | Pregn-5-en-20-on-3b-yl sulfurate | HMDB | | Pregn-5-en-20-on-3b-yl sulfuric acid | HMDB | | Pregn-5-en-20-one-3b-yl sulfate | HMDB | | Pregn-5-en-20-one-3b-yl sulphate | HMDB | | Pregnenolone 3-sulfate | HMDB | | Pregnenolone 3-sulphate | HMDB | | Pregnenolone 3b-sulfate | HMDB | | Pregnenolone 3b-sulphate | HMDB | | Pregnenolone hydrogen sulfate | HMDB | | Pregnenolone hydrogen sulphate | HMDB | | Pregnenolone monosulfate | HMDB | | Pregnenolone monosulphate | HMDB | | [(1S,2R,10S,11S,14S,15S)-14-Acetyl-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl]oxidanesulfonate | HMDB | | [(1S,2R,10S,11S,14S,15S)-14-Acetyl-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl]oxidanesulphonate | HMDB | | [(1S,2R,10S,11S,14S,15S)-14-Acetyl-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadec-7-en-5-yl]oxidanesulphonic acid | HMDB |
|
|---|
| Chemical Formula | C21H32O5S |
|---|
| Average Molecular Weight | 396.541 |
|---|
| Monoisotopic Molecular Weight | 396.197044824 |
|---|
| IUPAC Name | [(1S,2R,10S,11S,14S,15S)-14-acetyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-yl]oxidanesulfonic acid |
|---|
| Traditional Name | [(1S,2R,10S,11S,14S,15S)-14-acetyl-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-yl]oxidanesulfonic acid |
|---|
| CAS Registry Number | 1247-64-9 |
|---|
| SMILES | [H][C@@]12CC[C@H](C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC=C2CC(CC[C@]12C)OS(O)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C21H32O5S/c1-13(22)17-6-7-18-16-5-4-14-12-15(26-27(23,24)25)8-10-20(14,2)19(16)9-11-21(17,18)3/h4,15-19H,5-12H2,1-3H3,(H,23,24,25)/t15?,16-,17+,18-,19-,20-,21+/m0/s1 |
|---|
| InChI Key | DIJBBUIOWGGQOP-OZIWPBGVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sulfated steroids. These are sterol lipids containing a sulfate group attached to the steroid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Sulfated steroids |
|---|
| Direct Parent | Sulfated steroids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sulfated steroid skeleton
- 20-oxosteroid
- Pregnane-skeleton
- Oxosteroid
- Delta-5-steroid
- Sulfuric acid ester
- Sulfuric acid monoester
- Sulfate-ester
- Alkyl sulfate
- Organic sulfuric acid or derivatives
- Ketone
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Carbonyl group
- Organic oxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Endoplasmic reticulum
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-015c-1069000000-98638a32c64bd13693bb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0039000000-c29e72af13a8b1df901a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0092000000-48af0541d95515a42eca | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kai-5193000000-d981003b06fdf18508e3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0019000000-2cac349b15d130782aef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-1039000000-f63d28f7bec5ec72f437 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000t-6093000000-3f7db3bec91ec381b060 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-5d910d6c0e79d0a64f73 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f6t-1009000000-9f3fbb71720d2bb163cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9007000000-5ff2a20734539479696c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0019000000-d84db4f789aa355c64b2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zfs-0092000000-833a400098daed115a39 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k96-4691000000-5ee53c8e93e176521243 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, CD3OD, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CD3OD, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Killinger, Donald W.; Solomon, Samuel. Synthesis of pregnenolone sulfate, dehydroisoandrosterone sulfate, 17a-hydroxypregnenolone sulfate, and pregn-5-enetriol by the normal human adrenal gland. Journal of Clinical Endocrinology and Metabolism (1965), 25(2), 290-3. |
|---|