| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:37:38 UTC |
|---|
| Update Date | 2020-04-22 15:05:39 UTC |
|---|
| BMDB ID | BMDB0000858 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Monomethyl glutaric acid |
|---|
| Description | Monomethyl glutaric acid, also known as 4-(methoxycarbonyl)butyrate or 2-methyleneglutarate, belongs to the class of organic compounds known as fatty acid methyl esters. Fatty acid methyl esters are compounds containing a fatty acid that is esterified with a methyl group. They have the general structure RC(=O)OR', where R=fatty aliphatic tail or organyl group and R'=methyl group. Based on a literature review a significant number of articles have been published on Monomethyl glutaric acid. |
|---|
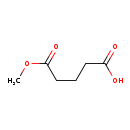
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-(Methoxycarbonyl)butyric acid | ChEBI | | 4-Methoxycarbonylbutanoic acid | ChEBI | | Monomethyl glutarate | ChEBI | | 4-(Methoxycarbonyl)butyrate | Generator | | 4-Methoxycarbonylbutanoate | Generator | | 2-Methyleneglutarate | HMDB | | 4-Carboxybutanoate | HMDB | | 4-Carboxybutanoic acid | HMDB | | 4-Carboxybutanoic acid methyl ester | HMDB | | 5-Methoxy-5-oxopentanoate | HMDB | | 5-Methoxy-5-oxopentanoic acid | HMDB | | Glutaric acid methyl ester | HMDB | | Glutaric acid methyl half ester | HMDB | | Glutaric acid monomethyl ester | HMDB | | Glutaric acid monomethylester | HMDB | | Methyl glutarate | HMDB | | Methyl glutarate,mono | HMDB | | Methyl hydrogen glutarate | HMDB | | mono-Methyl glutarate | HMDB | | Monomethyl ester OF glutarate | HMDB | | Monomethyl ester OF glutaric acid | HMDB | | Pentanedioate | HMDB | | Pentanedioic acid | HMDB | | Pentanedioic acid monomethyl ester | HMDB |
|
|---|
| Chemical Formula | C6H10O4 |
|---|
| Average Molecular Weight | 146.1412 |
|---|
| Monoisotopic Molecular Weight | 146.057908808 |
|---|
| IUPAC Name | 5-methoxy-5-oxopentanoic acid |
|---|
| Traditional Name | glutaric acid monomethyl ester |
|---|
| CAS Registry Number | 1501-27-5 |
|---|
| SMILES | COC(=O)CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H10O4/c1-10-6(9)4-2-3-5(7)8/h2-4H2,1H3,(H,7,8) |
|---|
| InChI Key | IBMRTYCHDPMBFN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty acid methyl esters. Fatty acid methyl esters are compounds containing a fatty acid that is esterified with a methyl group. They have the general structure RC(=O)OR', where R=fatty aliphatic tail or organyl group and R'=methyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acid esters |
|---|
| Direct Parent | Fatty acid methyl esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acid methyl ester
- Dicarboxylic acid or derivatives
- Methyl ester
- Carboxylic acid ester
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 150 - 151 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-9200000000-85284735b1db3deedb30 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0kmr-9500000000-e85caa1ba14ebb44d78a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, N/A (Annotated) | splash10-02t9-9500000000-e5caac5ace69543e05e2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, N/A (Annotated) | splash10-0006-9000000000-a3583f0b1b836865929f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, N/A (Annotated) | splash10-0006-9000000000-7367386631dcd87166f2 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - , negative | splash10-0f6t-0900000000-ef965d7cd6e886deba24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-1900000000-a524a85a4054ac2b0efb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fvs-9800000000-7819f1be449b417d63b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6u-9000000000-c1c7555cc2a29713bf98 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-802b6c932cf976c61882 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-5900000000-491b5fc13867ce9f0277 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-499af4f9df33e46ff879 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ftb-8900000000-618ad9c7d2c1450040f2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-9aac55cf5126b9148c0f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-f3389501842eee04de97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-02ta-9500000000-6a4e88db705e779f8813 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052f-9000000000-77161291067cec5ca054 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-a834df46ff737ff668d1 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|