| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:39:45 UTC |
|---|
| Update Date | 2020-04-22 15:06:17 UTC |
|---|
| BMDB ID | BMDB0001004 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
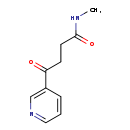
| Common Name | Oxoamide |
|---|
| Description | Oxoamide belongs to the class of organic compounds known as aryl alkyl ketones. These are ketones have the generic structure RC(=O)R', where R = aryl group and R'=alkyl group. Based on a literature review a significant number of articles have been published on Oxoamide. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| gamma-(3-Pyridyl)-gamma-oxo-N-methylbutyramide | MeSH | | 4-(3-Pyridyl)-4-oxo-N-methylbutyramide | HMDB | | 4-oxo-4-(3-Pyridyl)-N-methylbutanamide | HMDB | | N-Methyl-gamma-oxo-3-pyridinebutanamide | HMDB | | N-Methyl-4-oxo-4-(pyridin-3-yl)butanimidate | Generator, HMDB | | Oxoamide | MeSH |
|
|---|
| Chemical Formula | C10H12N2O2 |
|---|
| Average Molecular Weight | 192.2145 |
|---|
| Monoisotopic Molecular Weight | 192.089877638 |
|---|
| IUPAC Name | N-methyl-4-oxo-4-(pyridin-3-yl)butanamide |
|---|
| Traditional Name | N-methyl-4-oxo-4-(pyridin-3-yl)butanamide |
|---|
| CAS Registry Number | 713-05-3 |
|---|
| SMILES | CNC(=O)CCC(=O)C1=CN=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C10H12N2O2/c1-11-10(14)5-4-9(13)8-3-2-6-12-7-8/h2-3,6-7H,4-5H2,1H3,(H,11,14) |
|---|
| InChI Key | WOOSCPWOYYLIHS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aryl alkyl ketones. These are ketones have the generic structure RC(=O)R', where R = aryl group and R'=alkyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Aryl alkyl ketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aryl alkyl ketone
- Fatty amide
- N-acyl-amine
- Pyridine
- Fatty acyl
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Carboxamide group
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Organic nitrogen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -0.69 | LI,NY & GORROD,JW (1992) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a59-4900000000-b4127a0f5559e0c1ec06 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0900000000-e774a91f322b671f415a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2900000000-e5a5a9ae896687f43c25 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9700000000-4bb4da8a151d7770a636 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-f2612c39399a985683ff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01r6-3900000000-7f95fbc3d468a8d11e87 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056u-9300000000-9cebd2b0f8a67a81dda9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-b12a30a94a0bd68c8be3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00bc-9200000000-3d98bed4b61fbe2e42a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-9100000000-f798930c8874ff9f603e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-28e5a41c5b9b22fade09 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-2900000000-d37a2ff651bf32777825 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-9300000000-7028e1d53509091e52e9 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|