| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:40:12 UTC |
|---|
| Update Date | 2020-04-22 15:06:25 UTC |
|---|
| BMDB ID | BMDB0001036 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
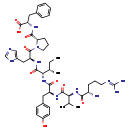
| Common Name | Angiotensin III |
|---|
| Description | Angiotensin III belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. Based on a literature review a significant number of articles have been published on Angiotensin III. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Des-asp-1-angiotensin II | HMDB | | Angiotensin II, des-asp | MeSH, HMDB | | Des asp angiotensin II | MeSH, HMDB | | Des aspartyl angiotensin II | MeSH, HMDB | | Des-asp angiotensin II | MeSH, HMDB | | Des-aspartyl-angiotensin II | MeSH, HMDB | | (2S)-2-({[(2S)-1-[(2S)-2-{[(2S,3S)-2-{[(2S)-2-{[(2S)-2-{[(2S)-2-amino-5-carbamimidamido-1-hydroxypentylidene]amino}-1-hydroxy-3-methylbutylidene]amino}-1-hydroxy-3-(4-hydroxyphenyl)propylidene]amino}-1-hydroxy-3-methylpentylidene]amino}-3-(1H-imidazol-5-yl)propanoyl]pyrrolidin-2-yl](hydroxy)methylidene}amino)-3-phenylpropanoate | Generator, HMDB | | Angiotensin III | MeSH |
|
|---|
| Chemical Formula | C46H66N12O9 |
|---|
| Average Molecular Weight | 931.0912 |
|---|
| Monoisotopic Molecular Weight | 930.50757177 |
|---|
| IUPAC Name | (2S)-2-{[(2S)-1-[(2S)-2-[(2S,3S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-5-[(diaminomethylidene)amino]pentanamido]-3-methylbutanamido]-3-(4-hydroxyphenyl)propanamido]-3-methylpentanamido]-3-(1H-imidazol-5-yl)propanoyl]pyrrolidin-2-yl]formamido}-3-phenylpropanoic acid |
|---|
| Traditional Name | (2S)-2-{[(2S)-1-[(2S)-2-[(2S,3S)-2-[(2S)-2-[(2S)-2-[(2S)-2-amino-5-[(diaminomethylidene)amino]pentanamido]-3-methylbutanamido]-3-(4-hydroxyphenyl)propanamido]-3-methylpentanamido]-3-(3H-imidazol-4-yl)propanoyl]pyrrolidin-2-yl]formamido}-3-phenylpropanoic acid |
|---|
| CAS Registry Number | 12687-51-3 |
|---|
| SMILES | CC[C@H](C)[C@H](NC(=O)[C@H](CC1=CC=C(O)C=C1)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCN=C(N)N)C(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C46H66N12O9/c1-5-27(4)38(57-40(61)33(21-29-15-17-31(59)18-16-29)53-42(63)37(26(2)3)56-39(60)32(47)13-9-19-51-46(48)49)43(64)54-34(23-30-24-50-25-52-30)44(65)58-20-10-14-36(58)41(62)55-35(45(66)67)22-28-11-7-6-8-12-28/h6-8,11-12,15-18,24-27,32-38,59H,5,9-10,13-14,19-23,47H2,1-4H3,(H,50,52)(H,53,63)(H,54,64)(H,55,62)(H,56,60)(H,57,61)(H,66,67)(H4,48,49,51)/t27-,32-,33-,34-,35-,36-,37-,38-/m0/s1 |
|---|
| InChI Key | QMMRCKSBBNJCMR-KMZPNFOHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Tyrosine or derivatives
- Phenylalanine or derivatives
- Histidine or derivatives
- Isoleucine or derivatives
- N-acyl-alpha-amino acid
- Valine or derivatives
- Proline or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- Alpha-amino acid amide
- 3-phenylpropanoic-acid
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- Imidazolyl carboxylic acid derivative
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Fatty amide
- N-acyl-amine
- Monocyclic benzene moiety
- Fatty acyl
- Benzenoid
- Heteroaromatic compound
- Tertiary carboxylic acid amide
- Pyrrolidine
- Imidazole
- Azole
- Carboxamide group
- Secondary carboxylic acid amide
- Guanidine
- Amino acid
- Amino acid or derivatives
- Azacycle
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Carboximidamide
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid
- Amine
- Organic nitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Organic oxide
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Primary amine
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06vi-5961232228-ea4adf55f419bbf3e0a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0200-6921010000-78b0faf8ce8050666bf0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0209-9410000000-bbebe48db6281a886339 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1000000092-4a7a50dd8f3ff61c013e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fr-7312022291-47ca9d35823f8d125113 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fu-9232011110-c5464e972b484b1210fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000100009-cb682999fd045f9fc4d9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03kc-5295211253-96684706f7b52c76d77e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03kd-6648180390-5726793f99bc63ef5611 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0223330329-7bdcba8cf45b54090ccb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06tr-3922011002-e608edceb9299f7d9e64 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dr-9500012201-3a78900784d621a6a389 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|