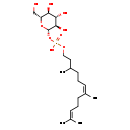| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:40:27 UTC |
|---|
| Update Date | 2020-04-22 15:06:30 UTC |
|---|
| BMDB ID | BMDB0001054 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Dolichyl b-D-glucosyl phosphate |
|---|
| Description | Dolichyl b-D-glucosyl phosphate, also known as dolichol monophosphate glucose, belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. Dolichyl b-D-glucosyl phosphate is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dolichyl b-D-glucosyl phosphoric acid | Generator | | Dolichol monophosphate glucose | HMDB | | Dolichyl b-delta-glucosyl phosphate | HMDB | | Dolichyl beta-D-glucosyl phosphate | HMDB | | Dolichyl beta-delta-glucosyl phosphate | HMDB | | Dolichyl monophosphate glucose | HMDB | | Dolichyl phosphate glucose | HMDB | | Dolichylphosphoryl glucose | HMDB |
|
|---|
| Chemical Formula | C21H39O9P |
|---|
| Average Molecular Weight | 466.5027 |
|---|
| Monoisotopic Molecular Weight | 466.233169358 |
|---|
| IUPAC Name | {[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}({[(6Z)-3,7,11-trimethyldodeca-6,10-dien-1-yl]oxy})phosphinic acid |
|---|
| Traditional Name | dolichyl phosphate glucose |
|---|
| CAS Registry Number | 220496-27-5 |
|---|
| SMILES | OC[C@H]1O[C@@H](OP(=O)(O)OCCC(C)CC\C=C(\C)CCC=C(C)C)[C@H](O)[C@@H](O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C21H39O9P/c1-14(2)7-5-8-15(3)9-6-10-16(4)11-12-28-31(26,27)30-21-20(25)19(24)18(23)17(13-22)29-21/h7,9,16-25H,5-6,8,10-13H2,1-4H3,(H,26,27)/b15-9-/t16?,17-,18-,19+,20-,21+/m1/s1 |
|---|
| InChI Key | RHJMCOLWMXJOGE-ZTZGTLKASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Farsesane sesquiterpenoid
- Hexose monosaccharide
- Monosaccharide phosphate
- Dialkyl phosphate
- Monosaccharide
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Alkyl phosphate
- Secondary alcohol
- Polyol
- Oxacycle
- Organoheterocyclic compound
- Primary alcohol
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9727600000-d38d6e1518a3a68d0771 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-014i-8414129000-afb66cc4f90fc385e62b | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2489500000-ee4f5c268ead36d45bbf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-6790000000-335bdfa9a636c87f56d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-5961000000-a91c7ccdc6413bbba7a7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gb9-2553900000-b96efe52d60699a8d378 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004l-9231100000-2bbc2deb52045a357ff5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-9000000000-e8e7a4fcaced53795fb5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0001900000-be2fd0839456e07b1706 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uxr-4109400000-9e442a9d85d51464dbe2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004j-9101000000-36de47c7bd711fdcd51b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-2223900000-79cd2a11d46e0223c333 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-08fr-9245000000-c2b6be31b362b77ce9b9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-9200000000-6fff84733fc82b56e1c2 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Rush, Jeffrey S.; Van Leyen, Klaus; Ouerfelli, Ouathek; Wolucka, Beata; Waechter, Charles J. Transbilayer movement of Glc-P-dolichol and its function as a glucosyl donor: protein-mediated transport of a water-soluble analog into sealed ER vesicles from pig brain. Glycobiology (1998), 8(12), 1195-1205. |
|---|