| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:41:53 UTC |
|---|
| Update Date | 2020-05-11 20:48:26 UTC |
|---|
| BMDB ID | BMDB0001165 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Keratan |
|---|
| Description | Keratan, also known as kerato sulfate or sulfate, keratan, belongs to the class of organic compounds known as oligosaccharide sulfates. These are carbohydrates containing between 3 and 9 sugar units, one of which bear one or more sulfate groups. Keratan is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
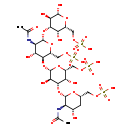
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Keratan 6'-sulfate | HMDB | | Keratan 6'-sulphate | HMDB | | Keratan sulfate | HMDB | | Keratan sulphate | HMDB | | Kerato 6'-sulfate | HMDB | | Kerato 6'-sulphate | HMDB | | Kerato sulfate | HMDB | | Kerato sulphate | HMDB | | N-[(2S,3R,4S,6S)-2-{[(2S,3R,4S,5S,6R)-3,5-dihydroxy-2-{[(2R,3S,4R,5R,6S)-4-hydroxy-5-[(1-hydroxyethylidene)amino]-2-[(sulfooxy)methyl]-6-{[(2R,3R,4S,5S,6R)-2,3,5-trihydroxy-6-[(sulfooxy)methyl]oxan-4-yl]oxy}oxan-3-yl]oxy}-6-[(sulfooxy)methyl]oxan-4-yl]oxy}-4-hydroxy-6-[(sulfooxy)methyl]oxan-3-yl]ethanimidate | HMDB | | N-[(2S,3R,4S,6S)-2-{[(2S,3R,4S,5S,6R)-3,5-dihydroxy-2-{[(2R,3S,4R,5R,6S)-4-hydroxy-5-[(1-hydroxyethylidene)amino]-2-[(sulphooxy)methyl]-6-{[(2R,3R,4S,5S,6R)-2,3,5-trihydroxy-6-[(sulphooxy)methyl]oxan-4-yl]oxy}oxan-3-yl]oxy}-6-[(sulphooxy)methyl]oxan-4-yl]oxy}-4-hydroxy-6-[(sulphooxy)methyl]oxan-3-yl]ethanimidate | HMDB | | N-[(2S,3R,4S,6S)-2-{[(2S,3R,4S,5S,6R)-3,5-dihydroxy-2-{[(2R,3S,4R,5R,6S)-4-hydroxy-5-[(1-hydroxyethylidene)amino]-2-[(sulphooxy)methyl]-6-{[(2R,3R,4S,5S,6R)-2,3,5-trihydroxy-6-[(sulphooxy)methyl]oxan-4-yl]oxy}oxan-3-yl]oxy}-6-[(sulphooxy)methyl]oxan-4-yl]oxy}-4-hydroxy-6-[(sulphooxy)methyl]oxan-3-yl]ethanimidic acid | HMDB | | Keratosulfate | HMDB | | Sulfate, keratan | HMDB |
|
|---|
| Chemical Formula | C28H48N2O32S4 |
|---|
| Average Molecular Weight | 1052.935 |
|---|
| Monoisotopic Molecular Weight | 1052.10730021 |
|---|
| IUPAC Name | {[(2S,4S,5R,6S)-5-acetamido-6-{[(2S,3R,4S,5S,6R)-2-{[(2R,3S,4R,5R,6S)-5-acetamido-4-hydroxy-2-[(sulfooxy)methyl]-6-{[(2R,3R,4S,5S,6R)-2,3,5-trihydroxy-6-[(sulfooxy)methyl]oxan-4-yl]oxy}oxan-3-yl]oxy}-3,5-dihydroxy-6-[(sulfooxy)methyl]oxan-4-yl]oxy}-4-hydroxyoxan-2-yl]methoxy}sulfonic acid |
|---|
| Traditional Name | [(2S,4S,5R,6S)-5-acetamido-6-{[(2S,3R,4S,5S,6R)-2-{[(2R,3S,4R,5R,6S)-5-acetamido-4-hydroxy-2-[(sulfooxy)methyl]-6-{[(2R,3R,4S,5S,6R)-2,3,5-trihydroxy-6-[(sulfooxy)methyl]oxan-4-yl]oxy}oxan-3-yl]oxy}-3,5-dihydroxy-6-[(sulfooxy)methyl]oxan-4-yl]oxy}-4-hydroxyoxan-2-yl]methoxysulfonic acid |
|---|
| CAS Registry Number | 69992-87-6 |
|---|
| SMILES | CC(=O)N[C@@H]1[C@@H](O)C[C@@H](COS(O)(=O)=O)O[C@H]1O[C@H]1[C@@H](O)[C@@H](COS(O)(=O)=O)O[C@@H](O[C@H]2[C@H](O)[C@@H](NC(C)=O)[C@H](O[C@@H]3[C@@H](O)[C@H](O)O[C@H](COS(O)(=O)=O)[C@@H]3O)O[C@@H]2COS(O)(=O)=O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C28H48N2O32S4/c1-8(31)29-15-11(33)3-10(4-52-63(40,41)42)56-26(15)62-24-18(35)13(6-54-65(46,47)48)58-28(21(24)38)60-22-14(7-55-66(49,50)51)59-27(16(19(22)36)30-9(2)32)61-23-17(34)12(5-53-64(43,44)45)57-25(39)20(23)37/h10-28,33-39H,3-7H2,1-2H3,(H,29,31)(H,30,32)(H,40,41,42)(H,43,44,45)(H,46,47,48)(H,49,50,51)/t10-,11-,12+,13+,14+,15+,16+,17-,18-,19+,20+,21+,22+,23-,24-,25+,26-,27-,28-/m0/s1 |
|---|
| InChI Key | KXCLCNHUUKTANI-RBIYJLQWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligosaccharide sulfates. These are carbohydrates containing between 3 and 9 sugar units, one of which bear one or more sulfate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Oligosaccharide sulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide sulfate
- N-acyl-alpha-hexosamine
- Glycosyl compound
- O-glycosyl compound
- Oxane
- Sulfuric acid monoester
- Sulfuric acid ester
- Alkyl sulfate
- Sulfate-ester
- Organic sulfuric acid or derivatives
- Acetamide
- Carboxamide group
- Hemiacetal
- Secondary alcohol
- Secondary carboxylic acid amide
- Oxacycle
- Carboxylic acid derivative
- Acetal
- Polyol
- Organoheterocyclic compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organic nitrogen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000f-9010060104-bd5beed3fb36ee571573 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02bo-3150491228-122cd61bbffb584c1ac0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000x-2150790013-12506dbbd36791439626 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ziu-9542136212-13f8482db4e33a98a64b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-007o-7164060369-735c66971e575bbf22f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-4459062000-0d3d89401963ff1ea5db | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-9000000102-47c8c6f51cd1bac1222e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0adi-2100203289-7780a86c7f9241ab8dbe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-4910003000-91dddc0febefc20b0779 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-9010000000-68550e6696781bcc899a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ow-8590030400-aed895d213a3d63b4712 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9010000000-8ef963b454130a6b7e68 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|