| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:45:49 UTC |
|---|
| Update Date | 2020-05-11 20:22:32 UTC |
|---|
| BMDB ID | BMDB0001427 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
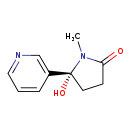
| Common Name | 5'-Hydroxycotinine |
|---|
| Description | 5'-Hydroxycotinine belongs to the class of organic compounds known as pyrrolidinylpyridines. Pyrrolidinylpyridines are compounds containing a pyrrolidinylpyridine ring system, which consists of a pyrrolidine ring linked to a pyridine ring. Based on a literature review very few articles have been published on 5'-Hydroxycotinine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-Hydroxycotinine | HMDB | | Allohydroxycotinine | HMDB |
|
|---|
| Chemical Formula | C10H12N2O2 |
|---|
| Average Molecular Weight | 192.2145 |
|---|
| Monoisotopic Molecular Weight | 192.089877638 |
|---|
| IUPAC Name | (5R)-5-hydroxy-1-methyl-5-(pyridin-3-yl)pyrrolidin-2-one |
|---|
| Traditional Name | 5-hydroxycotinine |
|---|
| CAS Registry Number | 61192-50-5 |
|---|
| SMILES | CN1C(=O)CC[C@@]1(O)C1=CN=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C10H12N2O2/c1-12-9(13)4-5-10(12,14)8-3-2-6-11-7-8/h2-3,6-7,14H,4-5H2,1H3/t10-/m1/s1 |
|---|
| InChI Key | BBNHNZGTKSWIHD-SNVBAGLBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrrolidinylpyridines. Pyrrolidinylpyridines are compounds containing a pyrrolidinylpyridine ring system, which consists of a pyrrolidine ring linked to a pyridine ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyrrolidinylpyridines |
|---|
| Direct Parent | Pyrrolidinylpyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrrolidinylpyridine
- Alkaloid or derivatives
- Pyrrolidone
- 2-pyrrolidone
- N-alkylpyrrolidine
- Pyrrolidine
- Tertiary carboxylic acid amide
- Heteroaromatic compound
- Lactam
- Carboxamide group
- Alkanolamine
- Carboxylic acid derivative
- Azacycle
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-4900000000-8fdc38303fa2e9d326fb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0096-8910000000-5a6063a4febbd48d8de4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-a2649b94dc708d963d16 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-1900000000-d93e8b5830f8c7b52206 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ini-9300000000-09c593e669cadd74a021 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-350e405fd056b470d809 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-e329ecf023780fbb4b78 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6r-9400000000-be7f33457d59944bfacb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-326a8bf022204233521d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001u-1900000000-bb5c51deec943ef81547 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0059-9300000000-c7ab8eb53e732eb149b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2900000000-dc09a61c7f2c3099543f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002f-4900000000-725ea1c3192b3356573d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056u-9600000000-5f4aedc4e9f9fde04d4c | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|