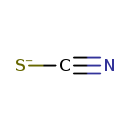Showing metabocard for Thiocyanate (BMDB0001453)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-09-30 22:46:11 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2020-05-20 23:09:43 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMDB ID | BMDB0001453 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Thiocyanate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Thiocyanate, also known as rhodanid or thiocyanic acid, belongs to the class of organic compounds known as thiocyanates. These are salts or esters of thiocyanic acid, with the general formula RSC#N (R=alkyl, aryl). Thiocyanate exists in all living species, ranging from bacteria to plants to humans. Based on a literature review a significant number of articles have been published on Thiocyanate. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | CNS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight | 58.082 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight | 57.975144695 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | cyanosulfanide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | thiocyanate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 302-04-5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [S-]C#N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/CHNS/c2-1-3/h3H/p-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | ZMZDMBWJUHKJPS-UHFFFAOYSA-M | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as thiocyanates. These are salts or esters of thiocyanic acid, with the general formula RSC#N (R=alkyl, aryl). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organosulfur compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Thiocyanates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Thiocyanates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ontology | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Expected but not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofunction | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Application | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biospecimen Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB0001453 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer Compound ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | FDB112173 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | C00000137 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | 8961 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Compound ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Thiocyanate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound | 9322 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 18022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Lang, Konrad. Thiocyanate formation in the animal body. II. Biochemische Zeitschrift (1933), 263 262-7. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Inorganic ion transport and metabolism
- Specific function:
- Together with MRPL18, acts as a mitochondrial import factor for the cytosolic 5S rRNA. Only the nascent unfolded cytoplasmic form is able to bind to the 5S rRNA (By similarity). Formation of iron-sulfur complexes and cyanide detoxification. Binds molecular oxygen and sulfur.
- Gene Name:
- TST
- Uniprot ID:
- P00586
- Molecular weight:
- 33296.0
Reactions
| 3-Mercaptopyruvic acid + Cyanide → Pyruvic acid + Thiocyanate | details |