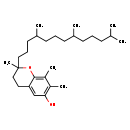
| DL-alpha-Tocopherol | Kegg |
| DL-a-Tocopherol | Generator |
| DL-Α-tocopherol | Generator |
| g-Tocopherol | Generator |
| Γ-tocopherol | Generator |
| 3,4-Dihydro-2,7,8-trimethyl-2-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-6-ol | HMDB |
| 7,8-Dimethyltocol | HMDB |
| 7,8-Dimethyltocolo-xylotocopherol | HMDB |
| Methyltocols | HMDB |
| O-Xylotocopherol | HMDB |
| Tocopherol | HMDB |
| Tocopherols | HMDB |
| Vitamin e gamma | HMDB |
| Bioweyxin | HMDB |
| e-Mulsin | HMDB |
| Embial | HMDB |
| GNR-Pharma brand OF tocopherol actetate | HMDB |
| Organon brand OF tocopherol acetate | HMDB |
| Schwarzhaupt brand OF tocopherol acetate | HMDB |
| Snow-e muscle, energy and feritility | HMDB |
| Spondyvit | HMDB |
| Tocolion | HMDB |
| Tocopherol rodisma-med brand | HMDB |
| Tocopherol worwag brand | HMDB |
| Togasan vitamin e | HMDB |
| Unique e | HMDB |
| Vibolex | HMDB |
| Vita-e | HMDB |
| Vitamin e dragees | HMDB |
| Atarost brand OF tocopherol acetate | HMDB |
| Biocur brand OF tocopherol acetate | HMDB |
| Cambridge laboratories brand OF tocopherol actetate | HMDB |
| Dal e | HMDB |
| Detulin | HMDB |
| e Vitamin e | HMDB |
| EVI-mirale, vitamin-e | HMDB |
| Elex verla | HMDB |
| Eplonat | HMDB |
| Richelet brand OF tocopherol acetate | HMDB |
| Roche nicholas brand OF tocopherol acetate | HMDB |
| Rodisma med brand OF tocopherol | HMDB |
| Sciencex brand OF tocopherol acetate | HMDB |
| Steigerwald brand OF tocopherol acetate | HMDB |
| Strathmann brand OF tocopherol | HMDB |
| Tocopherol grunwalder brand | HMDB |
| Tocopherol stadapharm brand | HMDB |
| Verla brand OF tocopherol | HMDB |
| Vit. e stada | HMDB |
| VitaPlus e | HMDB |
| Vitamin-e evi-mirale | HMDB |
| Vitamine e GNR | HMDB |
| Vitazell | HMDB |
| Worwag brand OF tocopherol | HMDB |
| Alcala brand OF tocopherol acetate | HMDB |
| Bio e | HMDB |
| Biopto-e | HMDB |
| e Vicotrat | HMDB |
| e-Ferol | HMDB |
| EUNOVA vitamin e | HMDB |
| Equivit e | HMDB |
| Evion | HMDB |
| Internation animal health brand OF tocopherol acetate | HMDB |
| MIP brand OF tocopherol acetate | HMDB |
| Sanum-kehlbeck brand OF tocopherol actetate | HMDB |
| Tocopherol infirmarius-rovit brand | HMDB |
| Tocopherol twardy brand | HMDB |
| Tocovital | HMDB |
| Vitamin-e dragees | HMDB |
| Wiedemann brand OF tocopherol | HMDB |
| Abortosan | HMDB |
| Arkopharma brand OF tocopherol acetate | HMDB |
| Dal vita brand OF vitamin e succinate | HMDB |
| Eu rho brand OF tocopherol | HMDB |
| Eusovit | HMDB |
| Infirmarius-rovit brand OF tocopherol | HMDB |
| Richtavit e | HMDB |
| Riemser brand OF tocopherol | HMDB |
| Tocopherol ausrichter brand | HMDB |
| Tocopherol balkanpharma brand | HMDB |
| Vitagutt vitamin e | HMDB |
| Vitamin e natur | HMDB |
| Vitamin e, togasan | HMDB |
| Ratiopharm brand OF tocopherol | HMDB |
| Aliud brand OF tocopherol | HMDB |
| Bottger brand OF tocopherol acetate | HMDB |
| Dermorelle | HMDB |
| e Ferol | HMDB |
| e-Vitamin-ratiopharm | HMDB |
| Heyl brand OF tocopherol acetate | HMDB |
| Kohler brand OF tocopherol | HMDB |
| Lasar | HMDB |
| Puncto e | HMDB |
| Rodisma-med brand OF tocopherol | HMDB |
| Sanum kehlbeck brand OF tocopherol actetate | HMDB |
| Scot tussin brand OF tocopherol acetate | HMDB |
| Stadapharm brand OF tocopherol | HMDB |
| Tocopa | HMDB |
| Tocopherol bayer | HMDB |
| Tocopherol grace brand | HMDB |
| Tocopherol ratiopharm brand | HMDB |
| Twardy brand OF tocopherol | HMDB |
| Uno vit | HMDB |
| Uno-vit | HMDB |
| Veyx brand OF tocopherol | HMDB |
| Vit e hydrosol | HMDB |
| Vita e | HMDB |
| Vitamin e al | HMDB |
| Wiedemann brand OF vitamin e succinate | HMDB |
| Ausrichter brand OF tocopherol | HMDB |
| Auxina e | HMDB |
| Balkanpharma brand OF tocopherol | HMDB |
| Blackmores brand OF tocopherol | HMDB |
| Dal-e | HMDB |
| Davitamon | HMDB |
| e-Vicotrat | HMDB |
| Ecoro | HMDB |
| Ephynal | HMDB |
| Grunwalder brand OF tocopherol | HMDB |
| ICN brand OF tocopherol acetate | HMDB |
| Mucos brand OF tocopherol acetate | HMDB |
| Tocopherol togal brand | HMDB |
| Tocopherol verla brand | HMDB |
| Togal brand OF tocopherol | HMDB |
| Troyapharm brand OF tocopherol acetate | HMDB |
| Vita-plus e | HMDB |
| Vitamin e sanum | HMDB |
| Vitamin e MP | HMDB |
| Vitamin emp | HMDB |
| Woelm brand OF tocopherol acetate | HMDB |
| Bayer brand OF tocopherol acetate | HMDB |
| GNR Pharma brand OF tocopherol actetate | HMDB |
| Grace brand OF tocopherol | HMDB |
| Malton e | HMDB |
| Merck brand OF tocopherol acetate | HMDB |
| Mowivit vitamin e | HMDB |
| Tocopherol wiedemann brand | HMDB |
| UnoVit | HMDB |
| Vitamin e evi mirale | HMDB |
| Vitamin e suspension | HMDB |
| Vitamin e, mowivit | HMDB |
| Vitamin e, vitagutt | HMDB |
| Vitamin e-MP | HMDB |
| Adisseo brand OF tocopherol | HMDB |
| Antioxidans e-hevert | HMDB |
| Aquasol e | HMDB |
| AstraZeneca brand OF tocopherol acetate | HMDB |
| Biosan | HMDB |
| Dal-vita brand OF vitamin e succinate | HMDB |
| Dragees, vitamin-e | HMDB |
| e Mulsin | HMDB |
| Hervert brand OF tocopherol acetate | HMDB |
| Hydrovit e | HMDB |
| Infirmarius rovit brand OF tocopherol | HMDB |
| Jenapharm brand OF tocopherol | HMDB |
| Kentucky brand OF tocopherol | HMDB |
| Micorvit e | HMDB |
| Sanavitan S | HMDB |
| Scot-tussin brand OF tocopherol acetate | HMDB |
| Tocopharm | HMDB |
| Tocopherol kentucky brand | HMDB |
| Vita plus e | HMDB |
| VitaE | HMDB |
| Medphano brand OF tocopherol acetate | HMDB |
| VitaminE evimirale | HMDB |