| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:47:47 UTC |
|---|
| Update Date | 2020-05-21 16:27:14 UTC |
|---|
| BMDB ID | BMDB0001568 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | trans-2-Octenoic acid |
|---|
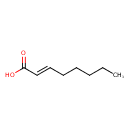
| Description | trans-2-Octenoic acid, also known as trans-2-octenoic acid, belongs to the class of organic compounds known as medium-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 4 and 12 carbon atoms. trans-2-Octenoic acid exists as a liquid, very hydrophobic, practically insoluble (in water), and relatively neutral molecule. trans-2-Octenoic acid exists in all eukaryotes, ranging from yeast to humans. trans-2-Octenoic acid participates in a number of enzymatic reactions, within cattle. In particular, trans-2-Octenoic acid can be biosynthesized from (R)-3-hydroxyoctanoic acid; which is mediated by the enzyme fatty acid synthase. dyhydrase domain. In addition, trans-2-Octenoic acid can be converted into caprylic acid through its interaction with the enzyme fatty acid synthase. enoyl reductase domain. In cattle, trans-2-octenoic acid is involved in the metabolic pathway called fatty acid biosynthesis pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (e)-2-Octenoic acid | ChEBI | | 2-Octenoic acid | ChEBI | | trans-alpha-Octenoic acid | ChEBI | | (e)-2-Octenoate | Generator | | 2-Octenoate | Generator | | trans-a-Octenoate | Generator | | trans-a-Octenoic acid | Generator | | trans-alpha-Octenoate | Generator | | trans-Α-octenoate | Generator | | trans-Α-octenoic acid | Generator | | trans-2-Octenoate | Generator | | (2E)-2-Octenoic acid | HMDB | | (2E)-Oct-2-enoic acid | HMDB | | trans-2-Octenoic acid | HMDB, ChEBI | | (e)-Oct-2-enoate | HMDB | | (e)-Oct-2-enoic acid | HMDB |
|
|---|
| Chemical Formula | C8H14O2 |
|---|
| Average Molecular Weight | 142.1956 |
|---|
| Monoisotopic Molecular Weight | 142.099379692 |
|---|
| IUPAC Name | (2E)-oct-2-enoic acid |
|---|
| Traditional Name | trans-2-octenoic acid |
|---|
| CAS Registry Number | 1871-67-6 |
|---|
| SMILES | CCCCC\C=C\C(O)=O |
|---|
| InChI Identifier | InChI=1S/C8H14O2/c1-2-3-4-5-6-7-8(9)10/h6-7H,2-5H2,1H3,(H,9,10)/b7-6+ |
|---|
| InChI Key | CWMPPVPFLSZGCY-VOTSOKGWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 4 and 12 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Medium-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain fatty acid
- Unsaturated fatty acid
- Straight chain fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Adiposome
- Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Liquid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 5 - 6 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 2.9 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0096-9000000000-801c8e0537a0c78cb64f | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0096-9000000000-801c8e0537a0c78cb64f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-054x-9100000000-674e58e1403584c37510 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05i3-9300000000-009fbcd018a04b967cd1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-0006-1900000000-b7bf23a0b3d2e169aaaf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-0006-3900000000-8f50ffbb66fd8d8a4093 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-0006-4900000000-9d8bc0c8f59ac419fbfd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - EI-B (HITACHI M-80B) , Positive | splash10-0096-9000000000-41aeee8c78cb838799da | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-3900000000-eabda8115bfbf3fc6fca | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001m-9200000000-38d844ded2c3da09c746 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-b1716f6f88db0d93c495 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-1900000000-5d702aa87f7d38dc2009 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0007-6900000000-ddfae845c01ce646730e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-9100000000-f2b136da6cc734a29fab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dl-0900000000-e92c8d4ba859e2c18a1a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0596-0900000000-15af8a5dc0356cd2f4b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9000000000-5f0acc9c148a535551fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9100000000-35fbf6036a26b499b040 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-a8d30352fd0b19afc3f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-45f40a1dbaae13a04dae | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, D2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, D2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, CD3OD, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Kameda, Kenji; Chikaki, Mariko; Morimoto, Chie; Jiang, Ming; Okuda, Hiromichi. Insulin-like actions of trans-10-hydroxy-2-decanoic acid and its related substances. Wakan Iyakugaku Zasshi (1996), 13(4), 456-457. |
|---|