| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:48:21 UTC |
|---|
| Update Date | 2020-04-22 15:08:55 UTC |
|---|
| BMDB ID | BMDB0001864 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 2-Ketohexanoic acid |
|---|
| Description | 2-Ketohexanoic acid, also known as alpha-ketocaproic acid or 2-oxohexanoate, belongs to the class of organic compounds known as medium-chain keto acids and derivatives. These are keto acids with a 6 to 12 carbon atoms long side chain. Based on a literature review a small amount of articles have been published on 2-Ketohexanoic acid. |
|---|
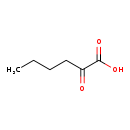
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Keto-N-caproic acid | ChEBI | | 2-oxo 6:0 | ChEBI | | 2-oxo C6:0 | ChEBI | | 2-Oxohexanoate | ChEBI | | alpha-Ketocaproic acid | ChEBI | | alpha-Ketohexanoic acid | ChEBI | | 2-Keto-N-caproate | Generator | | 2-Oxohexanoic acid | Generator | | a-Ketocaproate | Generator | | a-Ketocaproic acid | Generator | | alpha-Ketocaproate | Generator | | Α-ketocaproate | Generator | | Α-ketocaproic acid | Generator | | a-Ketohexanoate | Generator | | a-Ketohexanoic acid | Generator | | alpha-Ketohexanoate | Generator | | Α-ketohexanoate | Generator | | Α-ketohexanoic acid | Generator | | 2-Ketohexanoate | Generator | | 2-Ketocaproate | MeSH | | alpha-Ketocaproic acid, sodium salt | MeSH | | 2-Ketocaproic acid | HMDB | | 2-oxo-Hexanoate | HMDB | | 2-oxo-Hexanoic acid | HMDB | | 2-Ketohexanoic acid | ChEBI |
|
|---|
| Chemical Formula | C6H10O3 |
|---|
| Average Molecular Weight | 130.1418 |
|---|
| Monoisotopic Molecular Weight | 130.062994186 |
|---|
| IUPAC Name | 2-oxohexanoic acid |
|---|
| Traditional Name | 2-oxohexanoic acid |
|---|
| CAS Registry Number | 2492-75-3 |
|---|
| SMILES | CCCCC(=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H10O3/c1-2-3-4-5(7)6(8)9/h2-4H2,1H3,(H,8,9) |
|---|
| InChI Key | XNIHZNNZJHYHLC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain keto acids and derivatives. These are keto acids with a 6 to 12 carbon atoms long side chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Keto acids and derivatives |
|---|
| Sub Class | Medium-chain keto acids and derivatives |
|---|
| Direct Parent | Medium-chain keto acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain keto acid
- Alpha-keto acid
- Alpha-hydroxy ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-000j-8920000000-9e106a172aeaca6001ea | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-000j-8920000000-9e106a172aeaca6001ea | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-9000000000-e0cebba0b69620390c09 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-007a-9300000000-87b7d0f0e16ba9e212cb | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-01p9-9500000000-5145cab306a85b6e9d7f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-014i-9000000000-4cd8f41133a4d23b1d4c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-01qj-9100000000-caf6abf28104b7765cef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01qi-4900000000-702612249f0615c78f6b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0btm-9300000000-d02ff05157ccb2f8d416 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-dfcf44ed2c628b6b914a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-3900000000-d751dca8c7b118a0dea9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01s9-9500000000-c04f00087e8e89956b26 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-4a26ce3589a801c4bcef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052u-9000000000-a1430045d6ae5d2e494c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-9000000000-d6e0a51fd3a4a90d9ceb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-ed2b82d47ad4721d3a01 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-8be0a8820bd022f7267b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9000000000-761b5c64b393cb442dcc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-066u-9000000000-1763f75b095d5d429e5b | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H, 13C]-HSQC NMR Spectrum (2D, 600 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
|
|---|