| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:48:57 UTC |
|---|
| Update Date | 2020-05-11 20:48:54 UTC |
|---|
| BMDB ID | BMDB0001915 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
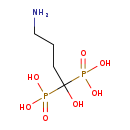
| Common Name | Alendronic acid |
|---|
| Description | Alendronic acid, also known as alendronate or acido alendronico, belongs to the class of organic compounds known as bisphosphonates. These are organic compounds containing two phosphonate groups linked together through a carbon atoms. Based on a literature review a significant number of articles have been published on Alendronic acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4-Amino-1-hydroxybutylidene)bisphosphonic acid | ChEBI | | Acide alendronique | ChEBI | | Acido alendronico | ChEBI | | Acidum alendronicum | ChEBI | | Alendronate | ChEBI | | (4-Amino-1-hydroxybutylidene)bisphosphonate | Generator | | 4-Amino-1-hydroxybutylidene 1,1-biphosphonate | MeSH | | Alendronate monosodium salt, trihydrate | MeSH | | Alendronate sodium | MeSH | | Aminohydroxybutane bisphosphonate | MeSH | | Fosamax | MeSH | | Alendronate sodium hydrate | HMDB | | Sodium, alendronate | MeSH, HMDB | | 4 amino 1 Hydroxybutylidene 1,1 biphosphonate | MeSH, HMDB | | Bisphosphonate, aminohydroxybutane | MeSH, HMDB |
|
|---|
| Chemical Formula | C4H13NO7P2 |
|---|
| Average Molecular Weight | 249.096 |
|---|
| Monoisotopic Molecular Weight | 249.016724799 |
|---|
| IUPAC Name | (4-amino-1-hydroxy-1-phosphonobutyl)phosphonic acid |
|---|
| Traditional Name | alendronate |
|---|
| CAS Registry Number | 66376-36-1 |
|---|
| SMILES | NCCCC(O)(P(O)(O)=O)P(O)(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H13NO7P2/c5-3-1-2-4(6,13(7,8)9)14(10,11)12/h6H,1-3,5H2,(H2,7,8,9)(H2,10,11,12) |
|---|
| InChI Key | OGSPWJRAVKPPFI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bisphosphonates. These are organic compounds containing two phosphonate groups linked together through a carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphonic acids and derivatives |
|---|
| Sub Class | Bisphosphonates |
|---|
| Direct Parent | Bisphosphonates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bisphosphonate
- Organophosphonic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Primary amine
- Organophosphorus compound
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9010000000-6b540b3cfe12455ee047 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-001i-9020000000-f4a0f6418448b2108371 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-014i-2930000000-99692afb4ed253251947 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-001i-0900000000-d2c07307d66e1c7d52e8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0kdl-9500000000-c82a6e6e898154627c7d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-0590000000-ed1b4585584330309b42 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uyi-4900000000-42101c55bda7e112b2af | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9330000000-8ac66f69531027a0950f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kb-2590000000-2c9bde6c398541c5cb71 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-017i-6950000000-d1f8822b01485883083d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003r-9000000000-7b5e5c1f9b218640040b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-0b08500c08babf97a735 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-005a-9050000000-04116055e92ba5f8a66f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03gi-9000000000-8274f2b488ab919859ff | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-eb3a6c3bae79932540c8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0190000000-7b88a34c07f93b4e4779 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9100000000-12b406fbd4d8f99b2d4d | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|