| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:49:55 UTC |
|---|
| Update Date | 2020-06-04 20:04:02 UTC |
|---|
| BMDB ID | BMDB0002013 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Butyrylcarnitine |
|---|
| Description | Butyrylcarnitine, also known as O-butanoylcarnitine, belongs to the class of organic compounds known as acyl carnitines. These are organic compounds containing a fatty acid with the carboxylic acid attached to carnitine through an ester bond. Thus, butyrylcarnitine is considered to be a fatty ester lipid molecule. Butyrylcarnitine is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Butyrylcarnitine exists in all eukaryotes, ranging from yeast to humans. Butyrylcarnitine is a potentially toxic compound. Butyrylcarnitine has been found to be associated with several diseases known as obesity, eosinophilic esophagitis, and pregnancy; also butyrylcarnitine has been linked to several inborn metabolic disorders including short chain acyl-coa dehydrogenase deficiency and very long chain acyl-coa dehydrogenase deficiency. |
|---|
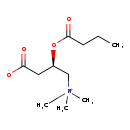
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3R)-3-(Butyryloxy)-4-(trimethylammonio)butanoate | ChEBI | | Butyryl-L-carnitine | ChEBI | | L-Carnitine butyryl ester | ChEBI | | N-Butyryl-L-(-)-carnitin | ChEBI | | O-Butanoyl-(R)-carnitine | ChEBI | | (3R)-3-(Butyryloxy)-4-(trimethylammonio)butanoic acid | Generator | | Butylcarnitine | HMDB | | O-Butanoyl-carnitine | HMDB | | O-Butanoylcarnitine | HMDB | | Butyrylcarnitine | ChEBI |
|
|---|
| Chemical Formula | C11H21NO4 |
|---|
| Average Molecular Weight | 231.292 |
|---|
| Monoisotopic Molecular Weight | 231.14705816 |
|---|
| IUPAC Name | (3R)-3-(butanoyloxy)-4-(trimethylazaniumyl)butanoate |
|---|
| Traditional Name | O-butanoylcarnitine |
|---|
| CAS Registry Number | 25576-40-3 |
|---|
| SMILES | CCCC(=O)O[C@H](CC([O-])=O)C[N+](C)(C)C |
|---|
| InChI Identifier | InChI=1S/C11H21NO4/c1-5-6-11(15)16-9(7-10(13)14)8-12(2,3)4/h9H,5-8H2,1-4H3/t9-/m1/s1 |
|---|
| InChI Key | QWYFHHGCZUCMBN-SECBINFHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acyl carnitines. These are organic compounds containing a fatty acid with the carboxylic acid attached to carnitine through an ester bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acid esters |
|---|
| Direct Parent | Acyl carnitines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acyl-carnitine
- Dicarboxylic acid or derivatives
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Carboxylic acid ester
- Carboxylic acid salt
- Carboxylic acid derivative
- Carboxylic acid
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic salt
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected and Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-000i-9000000000-a5dda83cb139267daf89 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-001r-8390000000-ceb4241ebded9660319e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-001i-0090000000-5c14cfc2ec68c45a3c6d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-001i-0090000000-ffb54886a9c9e0dd59c0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-000i-9000000000-45000c412d54fe872024 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-001r-6290000000-a53c2ce500986fed88fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-cf95ec9e05e8b6c1a98f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0019-9050000000-ebf3a8e1eb1d0ceaaeba | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9000000000-e9262cbaff8cb4ad0ba6 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| |
| Blood | Detected and Quantified | 0.2 +/- 0.1 uM | Not Specified | Not Specified | Normal | | details | | Blood | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Fibroblasts | Expected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Liver | Detected and Quantified | 0.36 +/- 0.08 nmol/g of tissue | Not Specified | Not Specified | Normal | | details | | Longissimus Thoracis Muscle | Detected and Quantified | 16 +/- 6 nmol/g of tissue | Not Specified | Not Specified | Normal | | details | | Milk | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Milk | Detected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Milk | Detected and Quantified | 4.4 +/- 0.2 uM | Not Specified | Not Specified | Normal | | details | | Milk | Detected and Quantified | 5.8 +/- 0.1 uM | Not Specified | Not Specified | Normal | | details | | Milk | Detected and Quantified | 6.6 +/- 0.1 uM | Not Specified | Not Specified | Normal | | details | | Milk | Detected and Quantified | 3.99 +/- 0.04 uM | Not Specified | Not Specified | Normal | | details | | Placenta | Expected but not Quantified | Not Quantified | Not Specified | Not Specified | Normal | | details | | Ruminal Fluid | Detected and Quantified | 0.05 +/- 0.02 uM | Not Specified | Not Specified | Normal | | details | | Semimembranosus Muscle | Detected and Quantified | 22 +/- 8 nmol/g of tissue | Not Specified | Not Specified | Normal | | details | | Testis | Detected and Quantified | 2.8 +/- 0.7 nmol/g of tissue | Not Specified | Not Specified | Normal | | details |
|
|---|
| General References | - Kurt J. Boudonck, Matthew W. Mitchell, Jacob Wulff and John A. Ryals (2009). Kurt J. Boudonck, Matthew W. Mitchell, Jacob Wulff and John A. Ryals. Characterization of the biochemical variability of bovine milk using metabolomics. Metabolomics (2009) 5:375?386. Metabolomics.
- Kurt J. Boudonck, Matthew W. Mitchell, Jacob Wulff, John A. Ryals (2009). Characterization of the biochemical variability of bovine milk using metabolomics. Metabolomics.
- A. Foroutan et al. (2019). A. Foroutan et al. The Chemical Composition of Commercial Cow's Milk (in preparation). Journal of Agricultural and Food Chemistry.
|
|---|