| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:53:23 UTC |
|---|
| Update Date | 2020-05-11 20:40:01 UTC |
|---|
| BMDB ID | BMDB0002271 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Imidazolepropionic acid |
|---|
| Description | Imidazolepropionic acid, also known as 4-imidazolylpropionate or deaminohistidine, belongs to the class of organic compounds known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. Imidazolepropionic acid exists in all living organisms, ranging from bacteria to humans. Based on a literature review a significant number of articles have been published on Imidazolepropionic acid. |
|---|
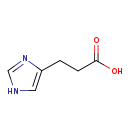
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1H-Imidazole-4-propanoic acid | ChEBI | | 3-(1H-Imidazol-4-yl)propionic acid | ChEBI | | 3-(Imidazol-4-yl)propanoic acid | ChEBI | | 3-(Imidazol-4-yl)propionic acid | ChEBI | | 4-Imidazolylpropanoic acid | ChEBI | | 4-Imidazolylpropionic acid | ChEBI | | Deaminohistidine | ChEBI | | Imidazolylpropionic acid | ChEBI | | 3-(1H-Imidazol-4-yl)propanoate | Kegg | | 1H-Imidazole-4-propanoate | Generator | | 3-(1H-Imidazol-4-yl)propionate | Generator | | 3-(Imidazol-4-yl)propanoate | Generator | | 3-(Imidazol-4-yl)propionate | Generator | | 4-Imidazolylpropanoate | Generator | | 4-Imidazolylpropionate | Generator | | Imidazolylpropionate | Generator | | 3-(1H-Imidazol-4-yl)propanoic acid | Generator | | Imidazolepropionate | Generator | | 3-(1H-Imidazol-4-yl)-propionate | HMDB | | 3-(1H-Imidazol-4-yl)-propionic acid | HMDB | | 5-Imidazolepropionate | HMDB | | 5-Imidazolepropionic acid | HMDB, MeSH | | deamino-Histidine | HMDB | | URO | HMDB | | Imidazole propionate | MeSH, HMDB | | Dihydrourocanate | Generator, HMDB | | 1H-Imidazole-4(5)-propanoate | Generator, HMDB | | 1H-Imidazole-4-propionic acid | HMDB | | 3-(1H-4-Imidazolyl)propanoic acid | HMDB | | 3-(1H-4-Imidazolyl)propionic acid | HMDB | | 3-(4-Imidazolyl)propanoic acid | HMDB | | 3-(4-Imidazolyl)propionic acid | HMDB | | 3-(Imidazol-4(5)-yl)propanoic acid | HMDB | | 3-(Imidazol-4(5)-yl)propionic acid | HMDB | | 5-(2-Carboxyethyl) imidazole | HMDB | | Dihydrourocanic acid | HMDB | | Dihydrourocanoic acid | HMDB | | Imidazole-4(5)-propanoic acid | HMDB | | Imidazole-4(5)-propionic acid | HMDB | | Imidazole-4-propanoic acid | HMDB | | Imidazole-4-propionic acid | HMDB | | Imidazolylpropanoic acid | HMDB | | beta-(5-Imidazolyl)propanoic acid | HMDB | | beta-(5-Imidazolyl)propionic acid | HMDB | | beta-Imidazolyl-4(5)-propanoic acid | HMDB | | beta-Imidazolyl-4(5)-propionic acid | HMDB | | β-(5-Imidazolyl)propanoic acid | HMDB | | β-(5-Imidazolyl)propionic acid | HMDB | | β-Imidazolyl-4(5)-propanoic acid | HMDB | | β-Imidazolyl-4(5)-propionic acid | HMDB |
|
|---|
| Chemical Formula | C6H8N2O2 |
|---|
| Average Molecular Weight | 140.1399 |
|---|
| Monoisotopic Molecular Weight | 140.05857751 |
|---|
| IUPAC Name | 3-(1H-imidazol-5-yl)propanoic acid |
|---|
| Traditional Name | 5-imidazolepropionic acid |
|---|
| CAS Registry Number | 1074-59-5 |
|---|
| SMILES | OC(=O)CCC1=CNC=N1 |
|---|
| InChI Identifier | InChI=1S/C6H8N2O2/c9-6(10)2-1-5-3-7-4-8-5/h3-4H,1-2H2,(H,7,8)(H,9,10) |
|---|
| InChI Key | ZCKYOWGFRHAZIQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Imidazoles |
|---|
| Direct Parent | Imidazolyl carboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Imidazolyl carboxylic acid derivative
- Heteroaromatic compound
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 206 - 208 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000y-9200000000-8af7c94edf62d10d7905 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dj-9200000000-466ffc1c8385b5811677 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - CE-QTOF-MS system (Agilent 7100 CE + 6550 QTOF) 20V, Positive | splash10-0006-0900000000-593be5cf10aba06261f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006x-1900000000-f813e755bd0c33989b99 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006t-9700000000-fc60958d4ee5d49335ca | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9000000000-c8f93079184c6ee4b5d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-2900000000-684a7e6823977a73c2b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000j-6900000000-3f4fc2398941bf5c7395 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05mo-9100000000-49b8e29531a2099a80ce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000b-9400000000-f2cb4a7574ec2ba4ce92 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-9100000000-8e37d715ddc9ef376bf7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-2ee2f80749be6e61f757 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-5900000000-bb071711eca6657a6d91 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9100000000-4bbf16992517da7c64f6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9000000000-fe8160b6c4b3de352e44 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|