| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:54:08 UTC |
|---|
| Update Date | 2020-04-22 15:10:39 UTC |
|---|
| BMDB ID | BMDB0002331 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
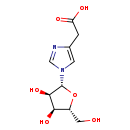
| Common Name | Imidazoleacetic acid riboside |
|---|
| Description | Imidazoleacetic acid riboside, also known as IAA-R or ribosylimidazole-4-acetic acid, belongs to the class of organic compounds known as imidazole ribonucleosides and ribonucleotides. These are organic compounds in which the C-1 of a ribosyl moiety is N-linked to an imidazole ring. Nucleotides have a phosphate group linked to the C5 carbon of the ribose (or deoxyribose) moiety. This class does not contain benzimidazole nucleosides and nucleotides. Based on a literature review very few articles have been published on Imidazoleacetic acid riboside. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Imidazoleacetate riboside | Generator | | IAA-R | MeSH | | Ribosylimidazole-4-acetic acid | MeSH | | Ribosylimidazole acetic acid | MeSH | | 1-b-D-Ribofuranosyl-imidazole-4-acetic acid | HMDB | | 1-beta-delta-Ribofuranosyl-imidazole-4-acetic acid | HMDB | | 1-Ribosylimidazole-4-acetic acid | HMDB | | Ribosylimidazoleacetate | HMDB | | (1-Ribosylimidazole)-4-acetic acid | Generator, HMDB | | Imidazoleacetic acid riboside | MeSH |
|
|---|
| Chemical Formula | C10H14N2O6 |
|---|
| Average Molecular Weight | 258.228 |
|---|
| Monoisotopic Molecular Weight | 258.08518619 |
|---|
| IUPAC Name | 2-{1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H-imidazol-4-yl}acetic acid |
|---|
| Traditional Name | ribosylimidazole acetic acid |
|---|
| CAS Registry Number | 29605-99-0 |
|---|
| SMILES | OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=NC(CC(O)=O)=C1 |
|---|
| InChI Identifier | InChI=1S/C10H14N2O6/c13-3-6-8(16)9(17)10(18-6)12-2-5(11-4-12)1-7(14)15/h2,4,6,8-10,13,16-17H,1,3H2,(H,14,15)/t6-,8-,9-,10-/m1/s1 |
|---|
| InChI Key | AHPWEWASPTZMEK-PEBGCTIMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as imidazole ribonucleosides and ribonucleotides. These are organic compounds in which the C-1 of a ribosyl moiety is N-linked to an imidazole ring. Nucleotides have a phosphate group linked to the C5 carbon of the ribose (or deoxyribose) moiety. This class does not contain benzimidazole nucleosides and nucleotides. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Imidazole ribonucleosides and ribonucleotides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Imidazole ribonucleosides and ribonucleotides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Imidazole ribonucleoside
- Glycosyl compound
- N-glycosyl compound
- Pentose monosaccharide
- Imidazolyl carboxylic acid derivative
- Monosaccharide
- N-substituted imidazole
- Azole
- Imidazole
- Heteroaromatic compound
- Tetrahydrofuran
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Monocarboxylic acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Organonitrogen compound
- Organooxygen compound
- Primary alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05bf-9420000000-7959b68020f3ef068c78 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0pl9-7655930000-6b1eb646515d38c64112 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6u-0970000000-3d8e5609d1b39e7b4801 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-0900000000-207d14b4f3f62f486e7b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-8900000000-9e8178d16dd80ec49376 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0590000000-f3fa710ee357a2d74111 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-2920000000-69577a44e601ae939560 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-8900000000-690bc05b5807ab94b7d5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-2890000000-e9f2aad1608ad0108975 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-8910000000-12c274c705c66cf2ef82 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zi3-9400000000-49e960c72a7917a8555e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0690000000-7654298b3d60f2399075 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a7i-3950000000-355655c064dd6d4aea3f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a7i-8900000000-e721a8e65963c98f6a7c | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|