| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 22:59:35 UTC |
|---|
| Update Date | 2020-04-22 15:11:03 UTC |
|---|
| BMDB ID | BMDB0002492 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
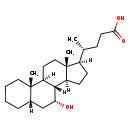
| Common Name | 7a-Hydroxy-5b-cholanic acid |
|---|
| Description | 7a-Hydroxy-5b-cholanic acid, also known as 3-deoxy-cdca or 7a-hydroxy-5b-cholan-24-Oate, belongs to the class of organic compounds known as monohydroxy bile acids, alcohols and derivatives. These are bile acids, alcohols or any of their derivatives bearing a hydroxyl group. 7a-Hydroxy-5b-cholanic acid is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Deoxy-cdca | ChEBI | | 3-DeoxyCDCA | ChEBI | | 7alpha-Hydroxy-5beta-cholan-24-Oic acid | ChEBI | | 7alpha-Hydroxy-5beta-cholanic acid | ChEBI | | 7a-Hydroxy-5b-cholan-24-Oate | Generator | | 7a-Hydroxy-5b-cholan-24-Oic acid | Generator | | 7alpha-Hydroxy-5beta-cholan-24-Oate | Generator | | 7Α-hydroxy-5β-cholan-24-Oate | Generator | | 7Α-hydroxy-5β-cholan-24-Oic acid | Generator | | 7a-Hydroxy-5b-cholanate | Generator | | 7alpha-Hydroxy-5beta-cholanate | Generator | | 7Α-hydroxy-5β-cholanate | Generator | | 7Α-hydroxy-5β-cholanic acid | Generator | | 7a-Hydroxy-5b-cholanoate | HMDB | | 7a-Hydroxy-5b-cholanoic acid | HMDB | | 3-Deoxychenodeoxycholate | HMDB | | (4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R)-9-Hydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoate | HMDB | | 7a-Hydroxy-5b-cholanic acid | Generator |
|
|---|
| Chemical Formula | C24H40O3 |
|---|
| Average Molecular Weight | 376.5726 |
|---|
| Monoisotopic Molecular Weight | 376.297745146 |
|---|
| IUPAC Name | (4R)-4-[(1S,2S,7S,9R,10R,11S,14R,15R)-9-hydroxy-2,15-dimethyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-yl]pentanoic acid |
|---|
| Traditional Name | 7a-hydroxy-5b-cholanate |
|---|
| CAS Registry Number | 28083-34-3 |
|---|
| SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@H](O)C[C@]4([H])CCCC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C24H40O3/c1-15(7-10-21(26)27)17-8-9-18-22-19(11-13-24(17,18)3)23(2)12-5-4-6-16(23)14-20(22)25/h15-20,22,25H,4-14H2,1-3H3,(H,26,27)/t15-,16+,17-,18+,19+,20-,22+,23+,24-/m1/s1 |
|---|
| InChI Key | JVMCMMXFADJQKU-MMSVWBHPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monohydroxy bile acids, alcohols and derivatives. These are bile acids, alcohols or any of their derivatives bearing a hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Monohydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monohydroxy bile acid, alcohol, or derivatives
- 7-hydroxysteroid
- Hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-1339000000-1748426d33d8bf6471ba | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0a4i-3223790000-4331610c0e43e0cee3fd | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0009000000-98131836749f94b76c63 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-1029000000-216b5ad98f22d08968d2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-7449000000-5df9cadfecbc48722ef2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-f317b23fea7a9c31f96a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6r-0009000000-501cf707675b3017b6f4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9006000000-8fb62cb5d050060eef79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-eccc9e9b8c78169f5313 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-0009000000-c70b5dcd12e8a885117c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-1019000000-6b99886aca5e81ddc24a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0019000000-f182918fe0e4a71a91ab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-6059000000-87535fcb28c834e2c8cd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-9240000000-b03351da33b1bdb913ff | View in MoNA |
|---|
|
|---|