| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:00:47 UTC |
|---|
| Update Date | 2020-04-22 15:11:08 UTC |
|---|
| BMDB ID | BMDB0002541 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Galactonolactone |
|---|
| Description | Galactonolactone belongs to the class of organic compounds known as lactones. These are cyclic esters of hydroxy carboxylic acids, containing a 1-oxacycloalkan-2-one structure, or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. Galactonolactone exists in all living organisms, ranging from bacteria to humans. Based on a literature review very few articles have been published on Galactonolactone. |
|---|
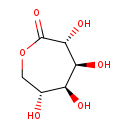
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Galactonolactone, (D)-isomer | MeSH | | Galactonolactone, (L)-isomer | MeSH | | D-galactono-1,4-Lactone | HMDB | | D-galactono-1,5-Lactone | HMDB | | D-galactono-8-Lactone | HMDB | | D-Galactonolactone | HMDB | | delta-galactono-1,4-Lactone | HMDB | | delta-galactono-1,5-Lactone | HMDB | | delta-galactono-8-Lactone | HMDB | | delta-Galactonolactone | HMDB | | gamma-D-Galactonolactone | HMDB | | gamma-delta-Galactonolactone | HMDB | | Galactonolactone | MeSH |
|
|---|
| Chemical Formula | C6H10O6 |
|---|
| Average Molecular Weight | 178.14 |
|---|
| Monoisotopic Molecular Weight | 178.047738052 |
|---|
| IUPAC Name | (3R,4S,5S,6R)-3,4,5,6-tetrahydroxyoxepan-2-one |
|---|
| Traditional Name | galactonolactone |
|---|
| CAS Registry Number | 2426-46-2 |
|---|
| SMILES | O[C@@H]1COC(=O)[C@H](O)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C6H10O6/c7-2-1-12-6(11)5(10)4(9)3(2)8/h2-5,7-10H,1H2/t2-,3+,4+,5-/m1/s1 |
|---|
| InChI Key | WTXGYGWMPUGBAL-MGCNEYSASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lactones. These are cyclic esters of hydroxy carboxylic acids, containing a 1-oxacycloalkan-2-one structure, or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Lactones |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Lactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Caprolactone
- Oxepane
- Carboxylic acid ester
- Lactone
- Secondary alcohol
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Oxacycle
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06rl-9400000000-9e4502053b3fe09f123e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0kou-3279300000-1418a34fa3efbbb205d1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-0900000000-685a5dbb4f8a7102dcc5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-2900000000-83611a0f9b640623ac24 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9200000000-fbfa10412acfeb6c4811 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1900000000-61baef021fc455ee67ea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-2900000000-9c6963bd735f92051d97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9100000000-4f71f72cc2972d6c238b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-4af21996c208dcba14ab | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fu-9500000000-ac5d2741d49de16a664a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0btc-9000000000-0a9b1531d89f4242cbcb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-1900000000-9622261df6835436c4f0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0abc-9200000000-3d5e0ea9ad8151fc6cc0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-8c8e168512fbb0f52fa3 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|