| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:01:42 UTC |
|---|
| Update Date | 2020-05-11 20:45:18 UTC |
|---|
| BMDB ID | BMDB0002818 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Alloxan |
|---|
| Description | Alloxan, also known as 5,6-dioxouracil or mesoxalylurea, belongs to the class of organic compounds known as pyrimidones. Pyrimidones are compounds that contain a pyrimidine ring, which bears a ketone. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. Based on a literature review a significant number of articles have been published on Alloxan. |
|---|
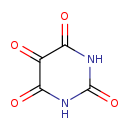
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,4,5,6(1H,3H)-Pyrimidinetetrone | ChEBI | | 2,4,5,6-Pyrimidinetetrone | ChEBI | | 2,4,5,6-Tetraoxohexahydropyrimidine | ChEBI | | 5,6-Dioxouracil | ChEBI | | 5-Oxobarbituric acid | ChEBI | | Alloxane | ChEBI | | Mesoxalylcarbamide | ChEBI | | Mesoxalylurea | ChEBI | | NSC 7169 | ChEBI | | Pyrimidinetetrone | ChEBI | | 5-Oxobarbitate | Generator | | 5-Oxobarbitic acid | Generator | | 2,4,5,6-Pyrimidintetron | HMDB | | 2,4,5,6-Pyrimidintetrone | HMDB | | 5-oxo-Barbiturate | HMDB | | 5-oxo-Barbituric acid | HMDB | | Alloxan 7169 | HMDB | | Alloxan tetrahydrat | HMDB | | Mesoxalyl-urea | HMDB |
|
|---|
| Chemical Formula | C4H2N2O4 |
|---|
| Average Molecular Weight | 142.0697 |
|---|
| Monoisotopic Molecular Weight | 142.001456562 |
|---|
| IUPAC Name | 1,3-diazinane-2,4,5,6-tetrone |
|---|
| Traditional Name | alloxan |
|---|
| CAS Registry Number | 50-71-5 |
|---|
| SMILES | O=C1NC(=O)C(=O)C(=O)N1 |
|---|
| InChI Identifier | InChI=1S/C4H2N2O4/c7-1-2(8)5-4(10)6-3(1)9/h(H2,5,6,8,9,10) |
|---|
| InChI Key | HIMXGTXNXJYFGB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrimidones. Pyrimidones are compounds that contain a pyrimidine ring, which bears a ketone. Pyrimidine is a 6-membered ring consisting of four carbon atoms and two nitrogen centers at the 1- and 3- ring positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Pyrimidones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidone
- Hydropyrimidine
- 2,5-dihydropyrimidine
- Ketone
- Carbonic acid derivative
- Cyclic ketone
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 256 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -1.84 | BIOBYTE (1995) |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9500000000-d176d0d6fcffbf493496 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-004i-9400000000-4493e387c163fd19672e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-056r-9000000000-ebb9b8d2b3db01ac1b30 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-01ox-9000000000-e390f807cb6126c81a8e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-1900000000-5b8617c49e887cf6346c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9400000000-2cc94355c95e93b2971f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ku-9000000000-dd466f209ed4ed32dfc6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-6900000000-4c1a6e3cf8a058b034cf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-ae29c061699f170b84a2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-1d9fa304c38627a77f7d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-4900000000-af3b61eae6a9fbb8e9a4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-90726b17dc36e29c5299 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-90726b17dc36e29c5299 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-3900000000-7554f9bede8cd74601d8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9200000000-2e8e42582aa24cbd48bc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9000000000-87926f5a9b3025440357 | View in MoNA |
|---|
|
|---|