| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:03:40 UTC |
|---|
| Update Date | 2020-04-22 15:12:00 UTC |
|---|
| BMDB ID | BMDB0003330 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Peroxyacetic acid uroporphyrin III |
|---|
| Description | Peroxyacetic acid uroporphyrin III belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. Based on a literature review very few articles have been published on Peroxyacetic acid uroporphyrin III. |
|---|
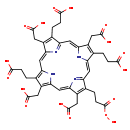
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Peroxyacetate uroporphyrin III | Generator | | C,C,C,3-Tetrakis(2-carboxyethyl)-C,C,C-tris(carboxymethyl)-21H,23H-porphine-2-ethaneperoxoate | HMDB | | C,C,C,3-Tetrakis(2-carboxyethyl)-C,C,C-tris(carboxymethyl)-21H,23H-porphine-2-ethaneperoxoic acid | HMDB | | 3-[20-(2-Carbonoperoxoylethyl)-9,14-bis(2-carboxyethyl)-5,10,15,19-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoate | Generator, HMDB |
|
|---|
| Chemical Formula | C40H38N4O17 |
|---|
| Average Molecular Weight | 846.7463 |
|---|
| Monoisotopic Molecular Weight | 846.22319581 |
|---|
| IUPAC Name | 3-[10-(2-carbonoperoxoylethyl)-14,19-bis(2-carboxyethyl)-5,9,15,20-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoic acid |
|---|
| Traditional Name | 3-[10-(2-carbonoperoxoylethyl)-14,19-bis(2-carboxyethyl)-5,9,15,20-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1³,⁶.1⁸,¹¹.1¹³,¹⁶]tetracosa-1,3,5,7,9,11(23),12,14,16,18(21),19-undecaen-4-yl]propanoic acid |
|---|
| CAS Registry Number | 163894-02-8 |
|---|
| SMILES | OOC(=O)CCC1=C(CC(O)=O)\C2=C\C3=C(CC(O)=O)C(CCC(O)=O)=C(N3)\C=C3/N=C(/C=C4\N\C(=C/C1=N2)C(CCC(O)=O)=C4CC(O)=O)C(CCC(O)=O)=C3CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C40H38N4O17/c45-33(46)5-1-17-21(9-36(51)52)29-14-27-18(2-6-34(47)48)22(10-37(53)54)30(43-27)15-28-19(3-7-35(49)50)23(11-38(55)56)31(44-28)16-32-24(12-39(57)58)20(4-8-40(59)61-60)26(42-32)13-25(17)41-29/h13-16,41,44,60H,1-12H2,(H,45,46)(H,47,48)(H,49,50)(H,51,52)(H,53,54)(H,55,56)(H,57,58)/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16- |
|---|
| InChI Key | LSLPFWLVSBAOHZ-UJJXFSCMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as porphyrins. Porphyrins are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
| Direct Parent | Porphyrins |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03gi-0000000690-81fcc6d562132e2bb41a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0159-0000000920-697b91910094d170854a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05rc-0000000900-f579ccfa74f67df0e092 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fai-0000000790-e0f0a58bd7c3bd8a763b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0000000930-ed5e3c630d9ff9352afb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001j-2000000900-b1e3127957eb0c4865eb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0560-0000000930-6500f8d00b29554762fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6u-0000000900-08597581c8693aba2ce7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6u-0000000900-7aa0763cdb5daec7a00a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0api-0000000900-f0d9fb6e901ed6ef5109 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-067i-0000000900-523df1cecafc4b31a814 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-0000000900-63a2976d405f5386316b | View in MoNA |
|---|
|
|---|