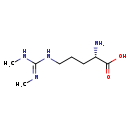| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:03:45 UTC |
|---|
| Update Date | 2020-05-11 20:40:29 UTC |
|---|
| BMDB ID | BMDB0003334 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Symmetric dimethylarginine |
|---|
| Description | Symmetric dimethylarginine, also known as N(g1),N(g2)-dimethylarginine or SDMA, belongs to the class of organic compounds known as arginine and derivatives. Arginine and derivatives are compounds containing arginine or a derivative thereof resulting from reaction of arginine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Based on a literature review a significant number of articles have been published on Symmetric dimethylarginine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-2-Amino-5-(n',n''-dimethylcarbamimidamido)pentanoic acid | ChEBI | | (S)-2-Amino-5-(n',n''-dimethylguanidino)pentanoic acid | ChEBI | | Guanidino-N(1),N(2)-dimethylarginine | ChEBI | | N(g1),N(g2)-Dimethylarginine | ChEBI | | N,N'-dimethylarginine | ChEBI | | N(3),N(4)-Dimethyl-L-arginine | ChEBI | | N(3),N(4)-Dimethylarginine | ChEBI | | N5-((Methylamino)(methylimino)methyl)-L-ornithine | ChEBI | | N(5)-(N,N'-dimethylamidino)-L-ornithine | ChEBI | | N(5)-(N,N'-dimethylcarbamimidoyl)-L-ornithine | ChEBI | | N(5)-[Bis(methylamino)methylene]-L-ornithine | ChEBI | | N(g),N'(g)-dimethyl-L-arginine | ChEBI | | N(g),N'(g)-dimethylarginine | ChEBI | | SDMA | ChEBI | | (2S)-2-Amino-5-(n',n''-dimethylcarbamimidamido)pentanoate | Generator | | (S)-2-Amino-5-(n',n''-dimethylguanidino)pentanoate | Generator | | N5-(N,N'-dimethylamidino)-L-ornithine | HMDB | | N5-[Bis(methylamino)methylene]-L-ornithine | HMDB | | NG,N'G-dimethyl-L-arginine | HMDB, MeSH | | NG,N'G-dimethylarginine | HMDB | | NG,NG'-dimethylarginine | HMDB | | Omega-N(g),n'(g)-dimethylarginine | MeSH, HMDB | | SDMA arginine | MeSH, HMDB |
|
|---|
| Chemical Formula | C8H18N4O2 |
|---|
| Average Molecular Weight | 202.2541 |
|---|
| Monoisotopic Molecular Weight | 202.14297584 |
|---|
| IUPAC Name | (2S)-2-amino-5-[(E)-N',N''-dimethylcarbamimidamido]pentanoic acid |
|---|
| Traditional Name | N3, N4-dimethylarginine |
|---|
| CAS Registry Number | 30344-00-4 |
|---|
| SMILES | CN\C(NCCC[C@H](N)C(O)=O)=N/C |
|---|
| InChI Identifier | InChI=1S/C8H18N4O2/c1-10-8(11-2)12-5-3-4-6(9)7(13)14/h6H,3-5,9H2,1-2H3,(H,13,14)(H2,10,11,12)/t6-/m0/s1 |
|---|
| InChI Key | HVPFXCBJHIIJGS-LURJTMIESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as arginine and derivatives. Arginine and derivatives are compounds containing arginine or a derivative thereof resulting from reaction of arginine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Arginine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Arginine or derivatives
- Alpha-amino acid
- L-alpha-amino acid
- Fatty acid
- Guanidine
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Carboximidamide
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Amine
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Primary aliphatic amine
- Imine
- Organic oxygen compound
- Carbonyl group
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kfx-9600000000-f6c986d30ea287579a78 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fkc-9720000000-0f297fd1183458956770 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-00di-9600000000-a9cb6507d9fe3ffb39aa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-053r-3910000000-1a8526e47c9af75d4cc9 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-0a59-9400000000-777d41b210dd091439b4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0pbi-1930000000-98ffa2f50a3e5ee90e10 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0abi-8900000000-eccaa3c6c71a7aab8dd3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9000000000-fd3f3045e508262e68be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-3390000000-ec3717ed507fec531202 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9520000000-06ed24b7ccb74ccd705b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dr-9000000000-40bdb35b370bc3317a55 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-2190000000-1ff257e4be06b9702e65 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9100000000-b9dfe8a8d6f891dcecbf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9000000000-926c9fc48c09f4030499 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ue9-1590000000-567e7d20ebbf052f8d39 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-2900000000-84e3189b175eab73e2c5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-c8ec00d7abbc06aa7e4c | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, experimental) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|