| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:05:44 UTC |
|---|
| Update Date | 2020-05-11 20:56:17 UTC |
|---|
| BMDB ID | BMDB0003654 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
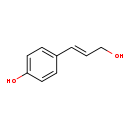
| Common Name | 4-Coumaryl alcohol |
|---|
| Description | 4-Coumaryl alcohol, also known as 4-hydroxycoumarin or 3-OHPP, belongs to the class of organic compounds known as cinnamyl alcohols. These are aromatic alcohols containing a 3-phenylprop-2-en-1-ol moiety. 4-Coumaryl alcohol is an extremely weak basic (essentially neutral) compound (based on its pKa). 4-Coumaryl alcohol exists in all living organisms, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (E)-4-Coumaryl alcohol | ChEBI, HMDB | | 4-Hydroxycinnamyl alcohol | ChEBI | | p-Coumaryl alcohol | ChEBI | | 3-(4-Hydroxyphenyl)-1-propane | HMDB | | 3-OHPP | HMDB | | 4-Coumarinol | HMDB | | 4-Hydroxy-2H-1-benzopyran-2-one | HMDB | | 4-Hydroxy-2H-chromen-2-one | HMDB | | 4-Hydroxycoumarin | HMDB | | 4-Hydroxycoumarine | HMDB | | Benzotetronate | HMDB | | Benzotetronic acid | HMDB | | Hydroxy coumarin | HMDB | | trans-3-(4'-Hydroxyphenyl)-2-propenoic acid | MeSH, HMDB | | trans-HPPA | MeSH, HMDB | | p-Hydroxycinnamic acid | MeSH, HMDB | | Para-coumaric acid | MeSH, HMDB | | 4-Coumaric acid, (E)-isomer | MeSH, HMDB | | 4-Hydroxycinnamic acid | MeSH, HMDB | | p-Coumaric acid | MeSH, HMDB | | 4-Coumaric acid | MeSH, HMDB | | (E)-4-Coumaroyl alcohol | ChEBI | | (E)-p-Coumaryl alcohol | HMDB | | 3-(p-Hydroxyphenyl)-2-propen-1-ol | HMDB | | 4-(3-Hydroxy-1-propen-1-yl)phenol | HMDB | | 4-Coumaryl alcohol | HMDB | | 4-Hydroxycinnamic alcohol | HMDB | | 4-[(1E)-3-Hydroxy-1-propenyl]phenol | HMDB | | Paracoumaryl alcohol | HMDB | | p-Coumaric alcohol | HMDB | | p-Cumaric alcohol | HMDB | | p-Hydroxycinnamic alcohol | HMDB | | p-Hydroxycinnamyl alcohol | HMDB | | trans-p-Coumaryl alcohol | HMDB |
|
|---|
| Chemical Formula | C9H10O2 |
|---|
| Average Molecular Weight | 150.1745 |
|---|
| Monoisotopic Molecular Weight | 150.068079564 |
|---|
| IUPAC Name | 4-[(1E)-3-hydroxyprop-1-en-1-yl]phenol |
|---|
| Traditional Name | paracoumaryl alcohol |
|---|
| CAS Registry Number | 1076-38-6 |
|---|
| SMILES | OC\C=C\C1=CC=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C9H10O2/c10-7-1-2-8-3-5-9(11)6-4-8/h1-6,10-11H,7H2/b2-1+ |
|---|
| InChI Key | PTNLHDGQWUGONS-OWOJBTEDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cinnamyl alcohols. These are aromatic alcohols containing a 3-phenylprop-2-en-1-ol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamyl alcohols |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Cinnamyl alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cinnamyl alcohol
- Styrene
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | 213.5 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0l1i-2900000000-35b232588497df392bcc | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00fu-9360000000-71f6e35237794477c995 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0900000000-d0aa38e43ffe69c65dce | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1900000000-f35b26b4169e49f89651 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4r-9600000000-feffeb0ec4d7bfd7e421 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-aa3fec540eb5ff87fecf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-926f68e15b016ddc1bf9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05nf-6900000000-53b966254d033e195810 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a59-0900000000-ef3175d0cc2e2c422a13 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ae9-4900000000-512eaa23bb6e2ec1bf3b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9200000000-835e5fb65c2a8f70004b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-0900000000-8d131986ef2454e28919 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0159-0900000000-c01ea2304d08f2059c07 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-3900000000-6bda1a3cbe19b1fee9f7 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|