| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:06:11 UTC |
|---|
| Update Date | 2020-04-22 15:12:46 UTC |
|---|
| BMDB ID | BMDB0003826 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
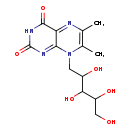
| Common Name | 6,7-Dimethyl-8-(1-D-ribityl)lumazine |
|---|
| Description | 6,7-Dimethyl-8-(1-D-ribityl)lumazine belongs to the class of organic compounds known as pteridines and derivatives. These are polycyclic aromatic compounds containing a pteridine moiety, which consists of a pyrimidine fused to a pyrazine ring to form pyrimido(4,5-b)pyrazine. 6,7-Dimethyl-8-(1-D-ribityl)lumazine is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). 6,7-Dimethyl-8-(1-D-ribityl)lumazine exists in all living organisms, ranging from bacteria to humans. In cattle, 6,7-dimethyl-8-(1-D-ribityl)lumazine is involved in the metabolic pathway called the riboflavin metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Deoxy-1-(3,4-dihydro-6,7-dimethyl-2,4-dioxo-8(2H)-pteridinyl)-ribitol | HMDB | | 1-Deoxy-1-[6,7-dimethyl-2,4-dioxo-3,4-dihydropteridin-8(2H)-yl]-D-ribitol | HMDB | | 6,7-Dimethyl-8-(1'-D-ribityl)lumazine | HMDB | | 6,7-Dimethyl-8-ribityllumazine | HMDB | | 6,7-Dimethyl-8-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]pteridine-2,4-dione | HMDB | | DMDRL | HMDB |
|
|---|
| Chemical Formula | C13H18N4O6 |
|---|
| Average Molecular Weight | 326.3052 |
|---|
| Monoisotopic Molecular Weight | 326.122634328 |
|---|
| IUPAC Name | 6,7-dimethyl-8-(2,3,4,5-tetrahydroxypentyl)-2,3,4,8-tetrahydropteridine-2,4-dione |
|---|
| Traditional Name | 6,7-dimethyl-8-(2,3,4,5-tetrahydroxypentyl)-3H-pteridine-2,4-dione |
|---|
| CAS Registry Number | 5118-16-1 |
|---|
| SMILES | CC1=C(C)N(CC(O)C(O)C(O)CO)C2=NC(=O)NC(=O)C2=N1 |
|---|
| InChI Identifier | InChI=1S/C13H18N4O6/c1-5-6(2)17(3-7(19)10(21)8(20)4-18)11-9(14-5)12(22)16-13(23)15-11/h7-8,10,18-21H,3-4H2,1-2H3,(H,16,22,23) |
|---|
| InChI Key | SXDXRJZUAJBNFL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pteridines and derivatives. These are polycyclic aromatic compounds containing a pteridine moiety, which consists of a pyrimidine fused to a pyrazine ring to form pyrimido(4,5-b)pyrazine. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Pteridines and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pteridine
- Pyrimidone
- Pyrazine
- Pyrimidine
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Secondary alcohol
- Polyol
- Azacycle
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary alcohol
- Alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | -3.145 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0bt9-9031000000-9c630ca6d5cf3abd4fa7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0k92-2122193000-4fdebb2ae42933ee8a1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-1059000000-bb2238e34adbb8c00787 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-4191000000-b3b1e68b02bd73ae782a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-056r-4940000000-f6a2f6ffebaa7bc97464 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0089-3090000000-c6c55b4bb2cd8266bc7e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9100000000-102fea61448a18c76296 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-d5a60b46dbd297e60a53 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, H2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|