| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:09:19 UTC |
|---|
| Update Date | 2020-04-22 15:13:45 UTC |
|---|
| BMDB ID | BMDB0004362 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 4-Hydroxynonenal |
|---|
| Description | 4-Hydroxynonenal, also known as HNE, belongs to the class of organic compounds known as fatty alcohols. These are aliphatic alcohols consisting of a chain of a least six carbon atoms. Thus, 4-hydroxynonenal is considered to be a fatty aldehyde lipid molecule. 4-Hydroxynonenal is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. 4-Hydroxynonenal is a potentially toxic compound. |
|---|
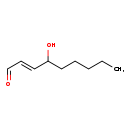
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (e)-4-Hydroxy-2-nonenal | ChEBI | | 4-Hydroxy-2,3-trans-nonenal | ChEBI | | HNE | ChEBI | | trans-4-Hydroxy-2-nonenal | ChEBI | | 4-Hydroxy-2,3-nonenal | HMDB | | 4-Hydroxy-2-nonenal | HMDB | | 4-Hydroxynon-2-enal | HMDB | | 4-Hydroxynonen-2-al | HMDB | | 4-HNE CPD | HMDB | | 4-Hydroxy-2-nonenal, (e)-isomer | HMDB | | 4-Hydroxynonenal | ChEBI |
|
|---|
| Chemical Formula | C9H16O2 |
|---|
| Average Molecular Weight | 156.2221 |
|---|
| Monoisotopic Molecular Weight | 156.115029756 |
|---|
| IUPAC Name | (2E)-4-hydroxynon-2-enal |
|---|
| Traditional Name | trans-4-hydroxy-2-nonenal |
|---|
| CAS Registry Number | 75899-68-2 |
|---|
| SMILES | CCCCCC(O)\C=C\C=O |
|---|
| InChI Identifier | InChI=1S/C9H16O2/c1-2-3-4-6-9(11)7-5-8-10/h5,7-9,11H,2-4,6H2,1H3/b7-5+ |
|---|
| InChI Key | JVJFIQYAHPMBBX-FNORWQNLSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty alcohols. These are aliphatic alcohols consisting of a chain of a least six carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty alcohols |
|---|
| Direct Parent | Fatty alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty alcohol
- Medium-chain aldehyde
- Enal
- Alpha,beta-unsaturated aldehyde
- Secondary alcohol
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aldehyde
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4r-9000000000-2b2e86f0d26bfcdbaa7c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a4i-9800000000-f25e4e5c5bfdea54d97c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-1900000000-9e0485f40ddea525bfb2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0abi-9700000000-14b48d43b52506ea875a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-c60e1ce771da41f3cef1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1900000000-963698def33862d38ed8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-3900000000-39790f81d0ebc18f6f71 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-a4ef5b05613d7c911528 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-c09fa78f1a3efa8c0e31 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ab9-5900000000-5f46d902d1ecb2e14a93 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aor-9100000000-e66e02441083fb8392a5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0007-9300000000-9c3d48d2b69a51fb7d63 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00r6-9000000000-fdc847c35b84e0c9d894 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-d7140511cfc2bca207a8 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|
| Synthesis Reference | Esterbauer, Hermann; Benedetti, Angelo; Lang, Johanna; Fulceri, Rosella; Fauler, Gunther; Comporti, Mario. Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidation. Biochimica et Biophysica Acta, Lipids and Lipid Metabolism (1986), 876(1), 154-66. |
|---|