Showing metabocard for N-Methylserotonin (BMDB0004369)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-09-30 23:09:22 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2020-05-21 16:27:05 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMDB ID | BMDB0004369 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | N-Methylserotonin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | N-Methylserotonin belongs to the class of organic compounds known as serotonins. Serotonins are compounds containing a serotonin moiety, which consists of an indole that bears an aminoethyl a position 2 and a hydroxyl group at position 5. N-Methylserotonin is possibly soluble (in water) and a very strong basic compound (based on its pKa). N-Methylserotonin and S-adenosylhomocysteine can be biosynthesized from serotonin and S-adenosylmethionine through its interaction with the enzyme indolethylamine N-methyltransferase. In cattle, N-methylserotonin is involved in the metabolic pathway called the tryptophan metabolism pathway. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
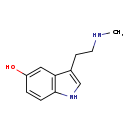
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C11H14N2O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight | 190.2417 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight | 190.11061308 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | 3-[2-(methylamino)ethyl]-1H-indol-5-ol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | N-methylserotonin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 1134-01-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | CNCCC1=CNC2=C1C=C(O)C=C2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C11H14N2O/c1-12-5-4-8-7-13-11-3-2-9(14)6-10(8)11/h2-3,6-7,12-14H,4-5H2,1H3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | ASUSBMNYRHGZIG-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as serotonins. Serotonins are compounds containing a serotonin moiety, which consists of an indole that bears an aminoethyl a position 2 and a hydroxyl group at position 5. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Indoles and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Tryptamines and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Serotonins | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ontology | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Expected but not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Origin |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biofunction | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Application | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biospecimen Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Abnormal Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB0004369 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer Compound ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | FDB017273 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | 132989 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Compound ID | C06212 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | 47668 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | N-Methylserotonin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | 7056 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound | 150885 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | 48294 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Somei, Masanori; Yamada, Fumio; Kurauchi, Takashi; Nagahama, Yoshiyuki; Hasegawa, Masakazu; Yamada, Koji; Teranishi, Sakiko; Sato, Haruhiko; Kaneko, Chikara. The chemistry of indoles. CIII. Simple syntheses of serotonin, N-methylserotonin, bufotenine, 5-m | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||