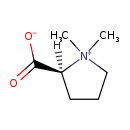| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:10:50 UTC |
|---|
| Update Date | 2020-05-11 20:40:58 UTC |
|---|
| BMDB ID | BMDB0004827 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Proline betaine |
|---|
| Description | Proline betaine, also known as stachydrine, belongs to the class of organic compounds known as proline and derivatives. Proline and derivatives are compounds containing proline or a derivative thereof resulting from reaction of proline at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. Proline betaine exists in all living organisms, ranging from bacteria to humans. Based on a literature review a significant number of articles have been published on Proline betaine. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (S)-2-Carboxylato-1,1-dimethylpyrrolidinium | ChEBI | | N,N-Dimethyl-L-proline | ChEBI | | Stachydrine | ChEBI | | L-Proline betaine | Kegg | | (2S)-2-Carboxy-1,1-dimethylpyrrolidinium | HMDB | | (S)-2-Carboxy-1,1-dimethylpyrrolidinium | HMDB | | Homostachydrine | HMDB | | Prestwick-08g03 | HMDB | | Dimethylproline | MeSH, HMDB | | Stachydrine chloride | MeSH, HMDB | | Stachydrine chloride, (S)-isomer | MeSH, HMDB | | Stachydrine, (+-)-isomer | MeSH, HMDB | | Proline betaine | ChEBI |
|
|---|
| Chemical Formula | C7H13NO2 |
|---|
| Average Molecular Weight | 143.1836 |
|---|
| Monoisotopic Molecular Weight | 143.094628665 |
|---|
| IUPAC Name | (2S)-1,1-dimethylpyrrolidin-1-ium-2-carboxylate |
|---|
| Traditional Name | stachydrine |
|---|
| CAS Registry Number | 471-87-4 |
|---|
| SMILES | C[N+]1(C)CCC[C@H]1C([O-])=O |
|---|
| InChI Identifier | InChI=1S/C7H13NO2/c1-8(2)5-3-4-6(8)7(9)10/h6H,3-5H2,1-2H3/t6-/m0/s1 |
|---|
| InChI Key | CMUNUTVVOOHQPW-LURJTMIESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as proline and derivatives. Proline and derivatives are compounds containing proline or a derivative thereof resulting from reaction of proline at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Proline and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Proline or derivatives
- Alpha-amino acid
- L-alpha-amino acid
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- N-alkylpyrrolidine
- Tetraalkylammonium salt
- Pyrrolidine
- Quaternary ammonium salt
- Carboxylic acid salt
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Organoheterocyclic compound
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Amine
- Carbonyl group
- Organic salt
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Detected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002e-9000000000-36bbb91342358293711a | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-9200000000-395432f0cf8795dd4cf7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-ea4782d2282bc28329c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0007-4900000000-75a3b0df6adc0100b9fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aou-9000000000-5df24b6079668b584f79 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-34921bec4b2fad07f505 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-3900000000-da3a14fcb5a4909b775e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000f-9000000000-7b04a861eee46ac86b66 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9500000000-135bbe696520934e172e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-9100000000-6d9ce7552b62c3e5d66c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-059w-9100000000-1648e60acdd97fc80a9d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-bf6e206b443f53ef90b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-bf6e206b443f53ef90b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-94164ee813c486cb0b96 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|