| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:14:25 UTC |
|---|
| Update Date | 2020-05-11 19:32:21 UTC |
|---|
| BMDB ID | BMDB0004988 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
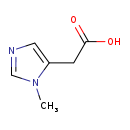
| Common Name | Pi-Methylimidazoleacetic acid |
|---|
| Description | Pi-Methylimidazoleacetic acid, also known as 1-methyl-imidazole-5-acetate or p-miaa, belongs to the class of organic compounds known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. Based on a literature review a significant number of articles have been published on Pi-Methylimidazoleacetic acid. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Methyl-imidazole-5-acetic acid | ChEBI | | pros-Methylimidazoleacetic acid | ChEBI | | 1-Methyl-imidazole-5-acetate | Generator | | pros-Methylimidazoleacetate | Generator | | Pi-methylimidazoleacetate | Generator | | p-MIAA | MeSH | | 1-Methyl-5-imidazoleacetic acid | HMDB | | Pi-methylimidazoleacetic acid | ChEBI | | 1-Methyl-5-imidazoleacetate | Generator, HMDB |
|
|---|
| Chemical Formula | C6H8N2O2 |
|---|
| Average Molecular Weight | 140.1399 |
|---|
| Monoisotopic Molecular Weight | 140.05857751 |
|---|
| IUPAC Name | 2-(1-methyl-1H-imidazol-5-yl)acetic acid |
|---|
| Traditional Name | P-miaa |
|---|
| CAS Registry Number | 4200-48-0 |
|---|
| SMILES | CN1C=NC=C1CC(O)=O |
|---|
| InChI Identifier | InChI=1S/C6H8N2O2/c1-8-4-7-3-5(8)2-6(9)10/h3-4H,2H2,1H3,(H,9,10) |
|---|
| InChI Key | UJCGYTCAMOHYCG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Imidazoles |
|---|
| Direct Parent | Imidazolyl carboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Imidazolyl carboxylic acid derivative
- N-substituted imidazole
- Heteroaromatic compound
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Azacycle
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-9400000000-03c4a0f73e55903dba95 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-009b-9200000000-7ced1cd26056a3e5788c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006x-0900000000-c71712049d7a61a77210 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dl-2900000000-1ecc98b5be58e80b8b90 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0jca-9200000000-f020fb0ff4ee468b3cd9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-3900000000-5a1f08ae8e10686a8019 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000j-8900000000-fed7fbafe3182a1f7952 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00b9-9300000000-7a7de3567ca8cd3454eb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-007p-5900000000-15a5a7246fde1324dace | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-007k-9300000000-ed1b73f3896312bc55a6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fr5-9000000000-e9a3c128ddd9fe68ed70 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9000000000-f99410898c25f7092925 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0092-9300000000-2e7146e29900dcf43df2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ue9-9100000000-bab2844da450045e3e7e | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|