| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:14:33 UTC |
|---|
| Update Date | 2020-05-11 20:52:02 UTC |
|---|
| BMDB ID | BMDB0005007 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Simvastatin |
|---|
| Description | Simvastatin, also known as zocor or MK-733, belongs to the class of organic compounds known as delta valerolactones. These are cyclic organic compounds containing an oxan-2- one moiety. Simvastatin is an extremely weak basic (essentially neutral) compound (based on its pKa). Simvastatin is a potentially toxic compound. |
|---|
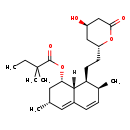
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,2-Dimethylbutyric acid, 8-ester with (4R,6R)-6-(2-((1S,2S,6R,8S,8ar)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl)tetrahydro-4-hydroxy-2H-pyran-2-one | ChEBI | | MK-733 | ChEBI | | Simvastatina | ChEBI | | Simvastatine | ChEBI | | Simvastatinum | ChEBI | | Zocor | ChEBI | | 2,2-Dimethylbutyrate, 8-ester with (4R,6R)-6-(2-((1S,2S,6R,8S,8ar)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl)tetrahydro-4-hydroxy-2H-pyran-2-one | Generator | | (+)-Simvastatin | HMDB | | Cholestat | HMDB | | Denan | HMDB | | Eucor | HMDB | | Kolestevan | HMDB | | Lipex | HMDB | | Lipinorm | HMDB | | Liponorm | HMDB | | Lipovas | HMDB | | Lodales | HMDB | | Modutrol | HMDB | | Nor-vastina | HMDB | | Pepstatin | HMDB | | Rechol | HMDB | | Simcor | HMDB | | Simovil | HMDB | | Simvastatin lactone | HMDB | | Simvotin | HMDB | | Sinvacor | HMDB | | Sinvascor | HMDB | | Sivastin | HMDB | | Statin | HMDB | | Synvinolin | HMDB | | Valemia | HMDB | | Velostatin | HMDB | | Zocord | HMDB |
|
|---|
| Chemical Formula | C25H38O5 |
|---|
| Average Molecular Weight | 418.5662 |
|---|
| Monoisotopic Molecular Weight | 418.271924326 |
|---|
| IUPAC Name | (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate |
|---|
| Traditional Name | simvastatin |
|---|
| CAS Registry Number | 79902-63-9 |
|---|
| SMILES | [H][C@]12[C@H](C[C@@H](C)C=C1C=C[C@H](C)[C@@H]2CC[C@@H]1C[C@@H](O)CC(=O)O1)OC(=O)C(C)(C)CC |
|---|
| InChI Identifier | InChI=1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1 |
|---|
| InChI Key | RYMZZMVNJRMUDD-HGQWONQESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as delta valerolactones. These are cyclic organic compounds containing an oxan-2- one moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Lactones |
|---|
| Sub Class | Delta valerolactones |
|---|
| Direct Parent | Delta valerolactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Delta_valerolactone
- Fatty acid ester
- Delta valerolactone
- Fatty acyl
- Oxane
- Dicarboxylic acid or derivatives
- Secondary alcohol
- Carboxylic acid ester
- Oxacycle
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fk9-9154200000-9a8f0d47976b67e5c9d5 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00b9-9137500000-2fdfacbca82ff764afb2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0229-0967000000-6c51c479e90818ec703b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-qTof , Positive | splash10-0229-0967000000-6c51c479e90818ec703b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0fya-0988400000-d9778b2f11696b81fb07 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-006w-0790000000-4f23e0376d85845e0964 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-05i1-0940000000-058f6298af54c05532ed | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0092-0910000000-6ecd838b2f6fddf67dad | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0597-0900000000-012d0d6fcabbd21d696d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-000e-0590000000-7f99e0b9531d4f42831a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0092-1960000000-e8429bbec985c5ecdd2d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0592-1910000000-dc09a51ca37fe4d24a5a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0abd-1900000000-9b55821f49270e5dd598 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-0536-1900000000-d8a41a97ccbdb30e5a5e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , positive | splash10-002f-2900000000-abe04d7f99c3eb0f2fcb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 60V, Positive | splash10-0abd-1900000000-78fbdab0700c9c2d0ea4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 45V, Positive | splash10-0592-1910000000-88e71bd3ee3ad70a99d1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 50V, Positive | splash10-0597-0900000000-093875a17b3bb042dc1b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0092-0910000000-6ecd838b2f6fddf67dad | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-05i1-0940000000-058f6298af54c05532ed | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0fya-0988400000-d9778b2f11696b81fb07 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-3028900000-ac9ec6a75c0ccc802808 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fr2-9156100000-30ee09c0a70d4a8c7b20 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kb-9120000000-4cb8e77d058b47cb3d82 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009500000-4449db04da972dd8d05f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01dj-4109100000-a14d60d17f23d1fb9872 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9131000000-24b3e278d457c517b414 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-0a4i-3900000000-d04758924bf88a90c017 | View in MoNA |
|---|
|
|---|
| Synthesis Reference | Willard, Alvin K.; Smith, Robert L.; Hoffman, William F. 6(R)-[2-(8-acyloxy-2-methyl-6-methyl (or hydrogen)-polyhydro-1-naphthyl)ethyl]-4(R)-hydroxy-3,4,5,6-tetrahydro-2H-pyran-2-ones, the hydroxy acid forms of these pyranones, salts and esters thereof, and a pharmaceutical antihypercholesterolemic composition containing them. Eur. Pat. Appl. (1981), 54 pp. CODEN: EPXXDW EP 33538 19810812 CAN 95:219968 AN 1981:619968 |
|---|