| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:15:17 UTC |
|---|
| Update Date | 2020-04-22 15:15:19 UTC |
|---|
| BMDB ID | BMDB0005083 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 8-Isoprostaglandin F2a |
|---|
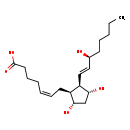
| Description | 8-Isoprostaglandin F2a, also known as 8-iso-PGF2a or 8-epi-PGF2alpha, belongs to the class of organic compounds known as prostaglandins and related compounds. These are unsaturated carboxylic acids consisting of a 20 carbon skeleton that also contains a five member ring, and are based upon the fatty acid arachidonic acid. Thus, 8-isoprostaglandin F2A is considered to be an eicosanoid. Based on a literature review a significant number of articles have been published on 8-Isoprostaglandin F2a. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 8-Epi-PGF2alpha | ChEBI | | 8-Epiprostaglandin F2alpha | ChEBI | | 8-Iso-PGF2a | ChEBI | | 8-Iso-PGF2alpha | ChEBI | | 8-Iso-PGF2alpha-III | ChEBI | | 8-IsoP | ChEBI | | 8-Isoprostaglandin F2alpha | ChEBI | | 8-Isoprostane | ChEBI | | 9,11,15-Trihydroxy-prosta-5,13-dien-1-Oic acid | ChEBI | | 8-Epi-prostaglandin F2alpha | Kegg | | 8-Epi-PGF2a | Generator | | 8-Epi-PGF2α | Generator | | 8-Epiprostaglandin F2a | Generator | | 8-Epiprostaglandin F2α | Generator | | 8-Iso-PGF2α | Generator | | 8-Iso-PGF2a-III | Generator | | 8-Iso-PGF2α-III | Generator | | 8-Isoprostaglandin F2α | Generator | | 9,11,15-Trihydroxy-prosta-5,13-dien-1-Oate | Generator | | 8-Epi-prostaglandin F2a | Generator | | 8-Epi-prostaglandin F2α | Generator | | (5Z,13E,15S)-9alpha,11alpha,15-Trihydroxy-8beta-prosta-5,13-dien-1-Oate | HMDB | | (5Z,13E,15S)-9alpha,11alpha,15-Trihydroxy-8beta-prosta-5,13-dien-1-Oic acid | HMDB | | 8-Epi PGF-2alpha | HMDB | | 9,11,15-Trihydroxy prosta-5,13-dien-1-Oate | HMDB | | 9,11,15-Trihydroxy prosta-5,13-dien-1-Oic acid | HMDB | | 9S,11R,15S-Trihydroxy-5Z,13E-prostadienoate | HMDB | | 9S,11R,15S-Trihydroxy-5Z,13E-prostadienoic acid | HMDB | | 9S,11R,15S-Trihydroxy-5Z,13E-prostadienoic acid-cyclo[8S,12R] | HMDB | | 15-F(2t)-IsoP | HMDB | | 15-F(2t)-Isoprostane | HMDB | | 8-F(2t)-Isoprostane | HMDB | | 8-Epi-PGF2 alpha | HMDB | | 8-Iso-PGF(2alpha) | HMDB | | Isoprostaglandin F2alpha type-III | HMDB | | 15-F2t-IsoP | HMDB | | 15-F2t-Isoprostane | HMDB | | 8-Isoprostaglandin F2a | Generator |
|
|---|
| Chemical Formula | C20H34O5 |
|---|
| Average Molecular Weight | 354.481 |
|---|
| Monoisotopic Molecular Weight | 354.240624198 |
|---|
| IUPAC Name | (5Z)-7-[(1S,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxyoct-1-en-1-yl]cyclopentyl]hept-5-enoic acid |
|---|
| Traditional Name | 8-isoprostane |
|---|
| CAS Registry Number | 27415-26-5 |
|---|
| SMILES | CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@H]1C\C=C/CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-19,21-23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16-,17+,18-,19+/m0/s1 |
|---|
| InChI Key | PXGPLTODNUVGFL-NAPLMKITSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as prostaglandins and related compounds. These are unsaturated carboxylic acids consisting of a 20 carbon skeleton that also contains a five member ring, and are based upon the fatty acid arachidonic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Prostaglandins and related compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Prostaglandin skeleton
- Long-chain fatty acid
- Hydroxy fatty acid
- Cyclopentanol
- Fatty acid
- Unsaturated fatty acid
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic oxide
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 2.183 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0019-6489000000-bf681ff653e664e9cde0 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-004i-9100177000-79583ccaf34002541a28 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0019000000-2c9767841fa4893c60b5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01bi-2297000000-8aa402b01c233708c42e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0103-9170000000-b6c0e28782ad3916af75 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0019000000-43645725f77edc02f608 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f7c-1049000000-d2fc6ca599de8bc5b9a5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9611000000-80d02fb42a0a241d8e2b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0019000000-ece58dc4c35634d7fe3f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-9255000000-c6aef7e365cba872a558 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mo-9300000000-552900f74505f7605b0b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-4f29fd22c6b0dfe3ac77 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udr-0098000000-06ede09931efe0fba78e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00el-9182000000-f1a4c9aba7f705d2868d | View in MoNA |
|---|
|
|---|