| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:15:18 UTC |
|---|
| Update Date | 2020-04-22 15:15:20 UTC |
|---|
| BMDB ID | BMDB0005084 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | N-Acetyl-leukotriene E4 |
|---|
| Description | N-Acetyl-leukotriene E4, also known as N acetyl LTE4 or N-ac-lte4, belongs to the class of organic compounds known as leukotrienes. These are eicosanoids containing a hydroxyl group attached to the aliphatic chain of an arachidonic acid. Leukotrienes have four double bonds, three (and only three) of which are conjugated. Thus, N-acetyl-leukotriene E4 is considered to be an eicosanoid. Based on a literature review a significant number of articles have been published on N-Acetyl-leukotriene E4. |
|---|
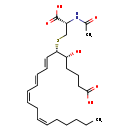
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N Acetyl lte4 | HMDB | | N-Ac-lte4 | HMDB | | N-Acetyl-lte4 | HMDB | | N-Acetylleukotriene e4 | HMDB | | NAcLTE4 | HMDB | | [5S-[5R*,6S*(s*),7E,9E,11Z,14Z]]-6-[[2-(acetylamino)-2-carboxyethyl]thio]-5-hydroxy-7,9,11,14-eicosatetraenoate | HMDB | | [5S-[5R*,6S*(s*),7E,9E,11Z,14Z]]-6-[[2-(acetylamino)-2-carboxyethyl]thio]-5-hydroxy-7,9,11,14-eicosatetraenoic acid | HMDB |
|
|---|
| Chemical Formula | C25H39NO6S |
|---|
| Average Molecular Weight | 481.645 |
|---|
| Monoisotopic Molecular Weight | 481.249808675 |
|---|
| IUPAC Name | (5R,6S,7E,9E,11Z,14Z)-6-{[(2S)-2-carboxy-2-acetamidoethyl]sulfanyl}-5-hydroxyicosa-7,9,11,14-tetraenoic acid |
|---|
| Traditional Name | (5R,6S,7E,9E,11Z,14Z)-6-{[(2S)-2-carboxy-2-acetamidoethyl]sulfanyl}-5-hydroxyicosa-7,9,11,14-tetraenoic acid |
|---|
| CAS Registry Number | 80115-95-3 |
|---|
| SMILES | CCCCC\C=C/C\C=C/C=C/C=C/[C@H](SC[C@@H](NC(C)=O)C(O)=O)[C@H](O)CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C25H39NO6S/c1-3-4-5-6-7-8-9-10-11-12-13-14-17-23(22(28)16-15-18-24(29)30)33-19-21(25(31)32)26-20(2)27/h7-8,10-14,17,21-23,28H,3-6,9,15-16,18-19H2,1-2H3,(H,26,27)(H,29,30)(H,31,32)/b8-7-,11-10-,13-12+,17-14+/t21-,22-,23+/m1/s1 |
|---|
| InChI Key | BGGYAYMMFYBWEX-KDFQUNDDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as leukotrienes. These are eicosanoids containing a hydroxyl group attached to the aliphatic chain of an arachidonic acid. Leukotrienes have four double bonds, three (and only three) of which are conjugated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Leukotrienes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Leukotriene
- Hydroxyeicosatetraenoic acid
- Long-chain fatty acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Cysteine or derivatives
- S-alkyl-l-cysteine
- Alpha-amino acid or derivatives
- Thia fatty acid
- Hydroxy fatty acid
- Dicarboxylic acid or derivatives
- Fatty acid
- Unsaturated fatty acid
- Acetamide
- Carboxamide group
- Secondary alcohol
- Secondary carboxylic acid amide
- Carboxylic acid derivative
- Carboxylic acid
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Carbonyl group
- Organic oxygen compound
- Organosulfur compound
- Organonitrogen compound
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | 3.698 | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kf-9105300000-d227e38dec5bec69bda8 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-01x3-9120317000-b51444a913ce4734d592 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03ej-0101900000-9e532e594641b71cd597 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1925800000-929e86b373a89cae7c7d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00m0-4649200000-ed5063fa0cc6771534fa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01q9-0204900000-061785437473c97d46da | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gx9-1429200000-49d7686b7a0717f1d17d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bvr-9711000000-79e44a9afb77aed2107c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0001900000-52c53f7cb7759d5f8279 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-2319000000-8c6c79f016bc4e0fd04f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-9101000000-f7b5bc6aacc809d9a4fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gwr-3907500000-0cdfd99a900a6547267e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0frl-3903000000-2eba6f1bf61972f39a50 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5c-2900000000-7608e7a899115dd2f9ec | View in MoNA |
|---|
|
|---|