| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:18:20 UTC |
|---|
| Update Date | 2020-04-22 15:16:16 UTC |
|---|
| BMDB ID | BMDB0005775 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Tetragastrin |
|---|
| Description | Tetragastrin, also known as CCK-4 or ccris 3246, belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. Based on a literature review a significant number of articles have been published on Tetragastrin. |
|---|
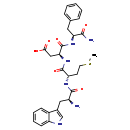
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| CCK-4 | ChEBI | | CCRIS 3246 | ChEBI | | Cholecystokinin tetrapeptide | ChEBI | | Cholecystokinin-4 | ChEBI | | Gastrin tetrapeptide | ChEBI | | Gastrin tetrapeptide amide | ChEBI | | L-TRP-L-Met-L-asp-L-phe-NH(2) | ChEBI | | L-Tryptophyl-L-methionyl-L-aspartyl-L-phenylalaninamide | ChEBI | | TRP-Met-asp-phe-NH(2) | ChEBI | | WMDF-NH(2) | ChEBI | | Carbobenzoxy-TRP-met-asp-phe-amide | HMDB | | Gatratet | HMDB | | L-Tryptophyl-L-methionyl-L-aspartylphenyl-alaninamide | HMDB | | 4, Cholecystokinin | MeSH, HMDB | | Cholecystokinin-tetrapeptide | MeSH, HMDB | | AOC-tetragastrin | MeSH, HMDB | | CCK (30-33) | MeSH, HMDB | | Cholecystokinin (30-33) | MeSH, HMDB | | Cholecystokinin 4 | MeSH, HMDB | | Tetrapeptide, gastrin | MeSH, HMDB | | AOC-tetrapeptide | MeSH, HMDB | | CKK-4 | MeSH, HMDB | | AOC tetragastrin | MeSH, HMDB | | AOC tetrapeptide | MeSH, HMDB |
|
|---|
| Chemical Formula | C29H36N6O6S |
|---|
| Average Molecular Weight | 596.698 |
|---|
| Monoisotopic Molecular Weight | 596.241703604 |
|---|
| IUPAC Name | (3S)-3-[(2S)-2-[(2S)-2-amino-3-(1H-indol-3-yl)propanamido]-4-(methylsulfanyl)butanamido]-3-{[(1S)-1-carbamoyl-2-phenylethyl]carbamoyl}propanoic acid |
|---|
| Traditional Name | (3S)-3-[(2S)-2-[(2S)-2-amino-3-(1H-indol-3-yl)propanamido]-4-(methylsulfanyl)butanamido]-3-{[(1S)-1-carbamoyl-2-phenylethyl]carbamoyl}propanoic acid |
|---|
| CAS Registry Number | 1947-37-1 |
|---|
| SMILES | CSCC[C@H](NC(=O)[C@@H](N)CC1=CNC2=CC=CC=C12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(N)=O |
|---|
| InChI Identifier | InChI=1S/C29H36N6O6S/c1-42-12-11-22(33-27(39)20(30)14-18-16-32-21-10-6-5-9-19(18)21)28(40)35-24(15-25(36)37)29(41)34-23(26(31)38)13-17-7-3-2-4-8-17/h2-10,16,20,22-24,32H,11-15,30H2,1H3,(H2,31,38)(H,33,39)(H,34,41)(H,35,40)(H,36,37)/t20-,22-,23-,24-/m0/s1 |
|---|
| InChI Key | RGYLYUZOGHTBRF-BIHRQFPBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Phenylalanine or derivatives
- Aspartic acid or derivatives
- Methionine or derivatives
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Triptan
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- 3-alkylindole
- Indole
- Indole or derivatives
- Aralkylamine
- Monocyclic benzene moiety
- Fatty amide
- N-acyl-amine
- Substituted pyrrole
- Fatty acyl
- Benzenoid
- Pyrrole
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Primary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Thioether
- Organoheterocyclic compound
- Dialkylthioether
- Sulfenyl compound
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Amine
- Hydrocarbon derivative
- Organic oxygen compound
- Primary aliphatic amine
- Organopnictogen compound
- Carbonyl group
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Organic oxide
- Primary amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-3900120000-5c94753fbda3e8b3ae94 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a4i-4911021000-ac75805ea7132e0d7b7c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Tetragastrin,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0522190000-9147056989212e59b65d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-0931010000-037fcba066e60e6dfe64 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pc3-2910000000-74754045897784a95545 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-4000090000-3fa10e322422852b0d0b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-9220030000-d93638019accce0fd9e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9220000000-b6b5d9f3b73597d1954d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0100090000-f757e4d3a27f3a4259bf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ogv-0921160000-7e0ca6a777425dc36500 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6x-3900000000-00beb9fe276ba345777e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000490000-6c1de053b22bf4b121a1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0w9u-3132490000-21608d2a37764b2671ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-6931000000-66c55eea268a548e064f | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|