| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:19:14 UTC |
|---|
| Update Date | 2020-04-22 15:16:32 UTC |
|---|
| BMDB ID | BMDB0005886 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | 19-Noretiocholanolone |
|---|
| Description | 19-Noretiocholanolone, also known as 19-norandrosterone or 3-hydroxyestran-17-one, belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. Based on a literature review a significant number of articles have been published on 19-Noretiocholanolone. |
|---|
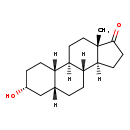
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 19-Norandrosterone, (3alpha,5alpha)-isomer | MeSH | | 19-Norandrosterone, (3beta,5beta)-isomer | MeSH | | 19-Norandrosterone, (3beta,5alpha,8alpha,9beta,10alpha,13alpha,14beta)-isomer | MeSH | | 19-Norandrosterone, (3alpha,5beta)-isomer | MeSH | | 19-Norandrosterone, (3beta)-isomer | MeSH | | 19-Norandrosterone, (3beta,5alpha)-isomer | MeSH | | 2,2,3,4,4,-D5-19-Nor-5alpha-androsterone | MeSH | | 19-Norandrosterone | MeSH | | 19-Noreoiandrosterone | MeSH | | 3-Hydroxyestran-17-one | MeSH | | (3a,5b)-3-Hydroxy-estran-17-one | HMDB | | 19-Noretiocholan-3a-ol-17-one | HMDB | | 3a-Hydroxy-5b-estran-17-one | HMDB | | 5b-Estran-3a-ol-17-one | HMDB | | 19-Noretiocholanolone | MeSH |
|
|---|
| Chemical Formula | C18H28O2 |
|---|
| Average Molecular Weight | 276.4137 |
|---|
| Monoisotopic Molecular Weight | 276.20893014 |
|---|
| IUPAC Name | (1R,2S,5R,7R,10R,11S,15S)-5-hydroxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-one |
|---|
| Traditional Name | (1R,2S,5R,7R,10R,11S,15S)-5-hydroxy-15-methyltetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadecan-14-one |
|---|
| CAS Registry Number | 33036-33-8 |
|---|
| SMILES | [H][C@@]12CCC(=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@]2([H])C[C@H](O)CC[C@]12[H] |
|---|
| InChI Identifier | InChI=1S/C18H28O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h11-16,19H,2-10H2,1H3/t11-,12-,13+,14-,15-,16+,18+/m1/s1 |
|---|
| InChI Key | UOUIARGWRPHDBX-DHMVHTBWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- Hydroxysteroid
- 3-alpha-hydroxysteroid
- 17-oxosteroid
- Oxosteroid
- Cyclic alcohol
- Ketone
- Secondary alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00l2-0390000000-3e9d3cc01211fe3f2e0c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0g1r-2269000000-927e4978162cfa2c72d4 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0090000000-cbc92b8361a51e9f9374 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05r0-0390000000-8f98ba1efcc73acea899 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f92-5980000000-22ddcd9c3181e54f339e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-020f4653853c30d0fb3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0090000000-fbcac4cd1c51b535f3d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4m-1190000000-8e893b5bd8d4088c8a09 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-7c08ae4a5c8bc21486d6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zi1-2980000000-16396bb89df457c641e7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ktb-2900000000-ee34ac41106cf13a9a3d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-4d3066b364d5c97a28e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0090000000-4d3066b364d5c97a28e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00fr-0090000000-e73157a0418447ebbe85 | View in MoNA |
|---|
|
|---|