| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:19:56 UTC |
|---|
| Update Date | 2020-04-22 15:16:46 UTC |
|---|
| BMDB ID | BMDB0006045 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Dityrosine |
|---|
| Description | Dityrosine belongs to the class of organic compounds known as lignans, neolignans and related compounds. These are plant products of low molecular weight formed primarily from oxidative coupling of two p-propylphenol moieties. They can also be described as micromolecules with two phenylpropanoid units coupled together. They can be attached in various manners, like C5-C5', C8-C8'. Most known natural lignans are oxidized at C9 and C9´ and, based upon the way in which oxygen is incorporated into the skeleton and on the cyclization patterns, a wide range of lignans of very different structural types can be formed. Based on a literature review a small amount of articles have been published on Dityrosine. |
|---|
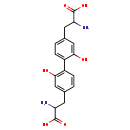
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Bityrosine | HMDB | | O,O'-dityrosine | HMDB | | O,O-Dityrosine | HMDB | | 2-Amino-3-[4'-(2-amino-2-carboxyethyl)-2,2'-dihydroxy-[1,1'-biphenyl]-4-yl]propanoate | Generator, HMDB |
|
|---|
| Chemical Formula | C18H20N2O6 |
|---|
| Average Molecular Weight | 360.3612 |
|---|
| Monoisotopic Molecular Weight | 360.132136382 |
|---|
| IUPAC Name | 2-amino-3-{4-[4-(2-amino-2-carboxyethyl)-2-hydroxyphenyl]-3-hydroxyphenyl}propanoic acid |
|---|
| Traditional Name | 2-amino-3-{4-[4-(2-amino-2-carboxyethyl)-2-hydroxyphenyl]-3-hydroxyphenyl}propanoic acid |
|---|
| CAS Registry Number | 980-21-2 |
|---|
| SMILES | NC(CC1=CC(O)=C(C=C1)C1=CC=C(CC(N)C(O)=O)C=C1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C18H20N2O6/c19-13(17(23)24)5-9-1-3-11(15(21)7-9)12-4-2-10(8-16(12)22)6-14(20)18(25)26/h1-4,7-8,13-14,21-22H,5-6,19-20H2,(H,23,24)(H,25,26) |
|---|
| InChI Key | FHOZGLMQYMAREG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lignans, neolignans and related compounds. These are plant products of low molecular weight formed primarily from oxidative coupling of two p-propylphenol moieties. They can also be described as micromolecules with two phenylpropanoid units coupled together. They can be attached in various manners, like C5-C5', C8-C8'. Most known natural lignans are oxidized at C9 and C9´ and, based upon the way in which oxygen is incorporated into the skeleton and on the cyclization patterns, a wide range of lignans of very different structural types can be formed. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lignans, neolignans and related compounds |
|---|
| Class | Not Available |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Lignans, neolignans and related compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylalanine or derivatives
- Neolignan skeleton
- Biphenyl
- 3-phenylpropanoic-acid
- Alpha-amino acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Phenol
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Benzenoid
- Amino acid or derivatives
- Amino acid
- Carboxylic acid
- Carboxylic acid derivative
- Primary aliphatic amine
- Primary amine
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Amine
- Organonitrogen compound
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00r6-4094000000-69cfcedbb99c8bb8cb22 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0ff0-5100039000-cb3449936fc7a3cc50b3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02t9-0039000000-ec7ae91410eca2c163ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0094000000-70dc4b0fd9a69a9afbb7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0090000000-6bfa163a49d5227bf160 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0009000000-baf07a29301dbcc652a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a59-2529000000-229b9b59e584d12c9814 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00e9-9811000000-f784f768398cc5e8fa4e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1029000000-99c9c87678bcb9643b95 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05tb-2096000000-157a4b079267ef2d7e78 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00vj-2191000000-ad98ef86b3ce473d2199 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01b9-0097000000-e6e5f8d807ad7ccd91a9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ba-0092000000-1f404f5753c0ce803bea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002g-2290000000-9895fb1cf10a8122083b | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|