| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:20:08 UTC |
|---|
| Update Date | 2020-04-22 15:16:50 UTC |
|---|
| BMDB ID | BMDB0006095 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
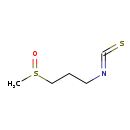
| Common Name | 3-Methylsulfinylpropyl isothiocyanate |
|---|
| Description | 3-Methylsulfinylpropyl isothiocyanate, also known as 3-isothiocyanatopropyl methyl sulfoxide or 1-isothiocyanato-3-(methylsulphinyl)propane, belongs to the class of organic compounds known as sulfoxides. Sulfoxides are compounds containing a sulfoxide functional group, with the structure RS(=O)R' (R,R' not H). Based on a literature review a significant number of articles have been published on 3-Methylsulfinylpropyl isothiocyanate. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Isothiocyanatopropyl methyl sulfoxide | ChEBI | | IMSP | ChEBI | | Methylsulfinylpropylisothiocyanate | ChEBI | | 3-Isothiocyanatopropyl methyl sulphoxide | Generator | | Methylsulfinylpropylisothiocyanic acid | Generator | | Methylsulphinylpropylisothiocyanate | Generator | | Methylsulphinylpropylisothiocyanic acid | Generator | | 3-Methylsulfinylpropyl isothiocyanic acid | Generator | | 3-Methylsulphinylpropyl isothiocyanate | Generator | | 3-Methylsulphinylpropyl isothiocyanic acid | Generator | | 1-Isothiocyanato-3-(methylsulphinyl)propane | MeSH | | 1-Isothiocyanato-3-(methylsulfinyl)propane | MeSH | | (R)-1-Isothiocyanato-3-(methylsulphinyl)propane | Generator |
|
|---|
| Chemical Formula | C5H9NOS2 |
|---|
| Average Molecular Weight | 163.261 |
|---|
| Monoisotopic Molecular Weight | 163.012555295 |
|---|
| IUPAC Name | 1-isothiocyanato-3-methanesulfinylpropane |
|---|
| Traditional Name | iberin |
|---|
| CAS Registry Number | 505-44-2 |
|---|
| SMILES | CS(=O)CCCN=C=S |
|---|
| InChI Identifier | InChI=1S/C5H9NOS2/c1-9(7)4-2-3-6-5-8/h2-4H2,1H3 |
|---|
| InChI Key | LELAOEBVZLPXAZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sulfoxides. Sulfoxides are compounds containing a sulfoxide functional group, with the structure RS(=O)R' (R,R' not H). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Sulfoxides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Sulfoxides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sulfoxide
- Isothiocyanate
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Sulfinyl compound
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0mdi-9100000000-cba8365c48cdd07cae16 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-1900000000-0f45d378074d3cee9fb1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-5900000000-5c92d444115013948405 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-ad018f5caa95c24942fe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-9400000000-3156b9c5eaa02f6c4c70 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9000000000-cbc7ae5d57c39a5f66be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08fr-9000000000-1a81c645e78474f5e1e0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-6900000000-67dd71397391c1848440 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9300000000-229345e9b4ce8990cbaf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-dbe2377aeefa575913e5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-4900000000-40d813da252f99b6c988 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zfr-9700000000-35cceb22e4f361385ddd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-9000000000-915fc15db1ff11b2de64 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|