| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:20:41 UTC |
|---|
| Update Date | 2020-05-21 16:29:06 UTC |
|---|
| BMDB ID | BMDB0006234 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
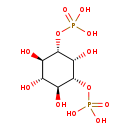
| Common Name | 1D-Myo-inositol 1,3-bisphosphate |
|---|
| Description | 1D-Myo-1d-1d-myo-inositol 1,3-bisphosphate, also known as 1d-myo-inositol 1,3-bisphosphate, belongs to the class of organic compounds known as inositol phosphates. Inositol phosphates are compounds containing a phosphate group attached to an inositol (or cyclohexanehexol) moiety. 1D-Myo-1d-1d-myo-inositol 1,3-bisphosphate is possibly soluble (in water) and an extremely weak basic (essentially neutral) compound (based on its pKa). 1D-Myo-1d-1d-myo-inositol 1,3-bisphosphate can be biosynthesized from inositol 1,3,4-trisphosphate through the action of the enzyme type ii inositol 3,4-bisphosphate 4-phosphatase. In cattle, 111d-myo-1d-1d-myo-inositol 1,3-bisphosphate is involved in the metabolic pathway called the inositol phosphate metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1D-Myo-inositol 1,3-bisphosphate | ChEBI | | Inositol 1,3-bisphosphate | ChEBI | | PHOSPHORIC ACID mono-(2,3,4,6-tetrahydroxy-5-phosphonooxy-cyclohexyl) ester | ChEBI | | Myo-inositol 1,3-bisphosphate | Kegg | | 1D-Myo-inositol 1,3-bisphosphoric acid | Generator | | Inositol 1,3-bisphosphoric acid | Generator | | PHOSPHate mono-(2,3,4,6-tetrahydroxy-5-phosphonooxy-cyclohexyl) ester | Generator | | Myo-inositol 1,3-bisphosphoric acid | Generator | | D-Myo-inositol 1,3-bisphosphoric acid | Generator | | D-myo-Inositol 1,3-bisphosphate | ChEBI | | Inositol 1,3-diphosphate | HMDB | | Inositol(1,3)bisphosphate | HMDB | | myo-Inositol 1,3-bis(dihydrogen phosphate) | HMDB | | myo-Inositol, 1,3-bis(dihydrogen phosphate) | HMDB |
|
|---|
| Chemical Formula | C6H14O12P2 |
|---|
| Average Molecular Weight | 340.1157 |
|---|
| Monoisotopic Molecular Weight | 339.996048936 |
|---|
| IUPAC Name | {[(1S,2R,3s,4S,5R,6s)-2,3,4,6-tetrahydroxy-5-(phosphonooxy)cyclohexyl]oxy}phosphonic acid |
|---|
| Traditional Name | [(1S,2R,3s,4S,5R,6s)-2,3,4,6-tetrahydroxy-5-(phosphonooxy)cyclohexyl]oxyphosphonic acid |
|---|
| CAS Registry Number | 103597-56-4 |
|---|
| SMILES | O[C@H]1[C@H](O)[C@@H](OP(O)(O)=O)[C@@H](O)[C@@H](OP(O)(O)=O)[C@@H]1O |
|---|
| InChI Identifier | InChI=1S/C6H14O12P2/c7-1-2(8)5(17-19(11,12)13)4(10)6(3(1)9)18-20(14,15)16/h1-10H,(H2,11,12,13)(H2,14,15,16)/t1-,2-,3+,4+,5+,6- |
|---|
| InChI Key | PUVHMWJJTITUGO-FICORBCRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as inositol phosphates. Inositol phosphates are compounds containing a phosphate group attached to an inositol (or cyclohexanehexol) moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Inositol phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Inositol phosphate
- Monoalkyl phosphate
- Cyclohexanol
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Secondary alcohol
- Polyol
- Organic oxide
- Hydrocarbon derivative
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9222000000-5c39a255ac5e58ce630c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-03dj-8612369000-ff5315111b8d0ebb3929 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-2029000000-f1ad7e189fc1a9d47074 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-2029000000-0ea5b95d380271ac0626 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9720000000-bb75401888953962682b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-4009000000-741e89441d559e5d1252 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9012000000-039a667e08561aac38c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-a3d3a318ae1d60f9714d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0009000000-409ec2d369e3f269fc61 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002r-5009000000-c245501f4c9988b006d9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-395fd651439ce717c08f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0009000000-0c0a6b1789da56429e47 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0019000000-48ee7745d7df03fb0ae6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9100000000-90d13222765893646b30 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 100 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 1000 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 200 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 300 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 400 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 500 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 600 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 700 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 800 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum (1D, 900 MHz, D2O, predicted) | Not Available | View in JSpectraViewer |
|---|
|
|---|