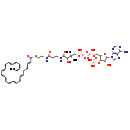| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-09-30 23:20:59 UTC |
|---|
| Update Date | 2020-04-22 15:17:06 UTC |
|---|
| BMDB ID | BMDB0006260 |
|---|
| Secondary Accession Numbers | |
|---|
| Metabolite Identification |
|---|
| Common Name | Trans-2-all-cis-6,9,12,15,18,21-tetracosaheptaenoyl-CoA |
|---|
| Description | Trans-2-all-cis-6,9,12,15,18,21-tetracosaheptaenoyl-CoA, also known as 2E,6Z,9Z,12Z,15Z,18Z,21Z-tetracosaheptaenoyl-CoA or tchpcoa, belongs to the class of organic compounds known as long-chain 2-enoyl coas. These are organic compounds containing a coenzyme A substructure linked to a long-chain 2-enoyl chain of 13 to 21 carbon atoms. Based on a literature review very few articles have been published on Trans-2-all-cis-6,9,12,15,18,21-tetracosaheptaenoyl-CoA. |
|---|
| Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2E,6Z,9Z,12Z,15Z,18Z,21Z-Tetracosaheptaenoyl-CoA | HMDB | | 2E,6Z,9Z,12Z,15Z,18Z,21Z-Tetracosaheptaenoyl-coenzyme A | HMDB | | TChpCoA | HMDB | | TCHPcoenzyme A | HMDB | | 4-({[({[5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-2-hydroxy-3,3-dimethyl-N-(2-{[2-(tetracosa-2,6,9,12,15,18,21-heptaenoylsulfanyl)ethyl]-C-hydroxycarbonimidoyl}ethyl)butanimidate | HMDB | | 4-({[({[5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-2-hydroxy-3,3-dimethyl-N-(2-{[2-(tetracosa-2,6,9,12,15,18,21-heptaenoylsulphanyl)ethyl]-C-hydroxycarbonimidoyl}ethyl)butanimidate | HMDB | | 4-({[({[5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-2-hydroxy-3,3-dimethyl-N-(2-{[2-(tetracosa-2,6,9,12,15,18,21-heptaenoylsulphanyl)ethyl]-C-hydroxycarbonimidoyl}ethyl)butanimidic acid | HMDB |
|
|---|
| Chemical Formula | C45H68N7O17P3S |
|---|
| Average Molecular Weight | 1104.05 |
|---|
| Monoisotopic Molecular Weight | 1103.360525926 |
|---|
| IUPAC Name | {[5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy({3-hydroxy-2,2-dimethyl-3-[(2-{[2-(tetracosa-2,6,9,12,15,18,21-heptaenoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy})phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional Name | [5-(6-aminopurin-9-yl)-4-hydroxy-2-({[hydroxy([hydroxy(3-hydroxy-2,2-dimethyl-3-[(2-{[2-(tetracosa-2,6,9,12,15,18,21-heptaenoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy)phosphoryl]oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxyphosphonic acid |
|---|
| CAS Registry Number | Not Available |
|---|
| SMILES | CCC=CCC=CCC=CCC=CCC=CCC=CCCC=CC(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OCC1OC(C(O)C1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N |
|---|
| InChI Identifier | InChI=1S/C45H68N7O17P3S/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-23-24-25-36(54)73-29-28-47-35(53)26-27-48-43(57)40(56)45(2,3)31-66-72(63,64)69-71(61,62)65-30-34-39(68-70(58,59)60)38(55)44(67-34)52-33-51-37-41(46)49-32-50-42(37)52/h5-6,8-9,11-12,14-15,17-18,20-21,24-25,32-34,38-40,44,55-56H,4,7,10,13,16,19,22-23,26-31H2,1-3H3,(H,47,53)(H,48,57)(H,61,62)(H,63,64)(H2,46,49,50)(H2,58,59,60) |
|---|
| InChI Key | NVOWZIBKQIWTDG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as long-chain 2-enoyl coas. These are organic compounds containing a coenzyme A substructure linked to a long-chain 2-enoyl chain of 13 to 21 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | Long-chain 2-enoyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Aminopyrimidine
- Alkyl phosphate
- Imidolactam
- Organic phosphoric acid derivative
- N-substituted imidazole
- Monosaccharide
- Pyrimidine
- Phosphoric acid ester
- Oxolane
- Heteroaromatic compound
- Azole
- Imidazole
- Carbothioic s-ester
- Amino acid or derivatives
- Thiocarboxylic acid ester
- Secondary alcohol
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organoheterocyclic compound
- Azacycle
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid derivative
- Oxacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Primary amine
- Alcohol
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Amine
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Ontology |
|---|
| Status | Expected but not Quantified |
|---|
| Origin | |
|---|
| Biofunction | Not Available |
|---|
| Application | Not Available |
|---|
| Cellular locations | - Cell membrane
- Cytoplasm
- Membrane
- Mitochondria
- Peroxisome
|
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-4901120100-7f8858f862870265518b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1901340000-853b4dd6837fd0c60a2e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1900010000-3c97d732115cd8250271 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001r-5904240500-22bb029d3d85853ee2e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-5903220100-772bb7cd4e88d2bcf648 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057i-5900100000-546ffadf4723438fea06 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-0439aa63591f912522a2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ufr-5902302410-4954f58fe11b2b17b12f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9001200300-c9c4e2386b2ac1008f1c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1900000010-257e43a8140b479f98aa | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0019-8600100109-2f7a0daf8a9b90f016a7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0000390000-b6aaa0f38851c8ad4d71 | View in MoNA |
|---|
|
|---|